A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- Which is correct order of bond strength ?
Text Solution
|
- Mass of H(2)O present in air in 10 lit. closed vessel with 80% relativ...
Text Solution
|
- What is the formal charge on nitrogen in NO(3)^(-) ?
Text Solution
|
- For which of the following reactions average molar mass at any progres...
Text Solution
|
- What is the shape of [F(2)IO(2)]^(-) ion ?
Text Solution
|
- U(avg) speed of O(2) at pi xx 10 bar pressure in a 8 litre container c...
Text Solution
|
- Which of the hexa -atomic species contains two lone pair on central at...
Text Solution
|
- Volume of 0.5 M Ba (OH)(2) require to neutralize 100 ml 0.8 M H(3) PO(...
Text Solution
|
- Which of the following diagram / statement is correct for the hybrid o...
Text Solution
|
- Concentration of Cl^(-) ions in a solution obtained by mixing 600 ml o...
Text Solution
|
- Compound which has maximum peroxide linkage is -
Text Solution
|
- If 61.25 gm of K ClO(3) reacts with excess of red phosphorus, what mas...
Text Solution
|
- Read the following statement regarding O(3) molecule (i) Each oxygen...
Text Solution
|
- Hydrogen cyanide is produced industrially from the reaction of gaseous...
Text Solution
|
- Ion which has maximum tendency to accept the electrons -
Text Solution
|
- At what temperature would CO(2) molecule have an rms speed equal to H(...
Text Solution
|
- Which of the following molecule does not exist in 3- D covalent solid ...
Text Solution
|
- If 0.2 mol of O(2) vapours can effuse from an opening in a heated vess...
Text Solution
|
- The correct order of boiling point is :
Text Solution
|
- At an under water depth of 400 feet, the pressure is 10 atm. What shou...
Text Solution
|
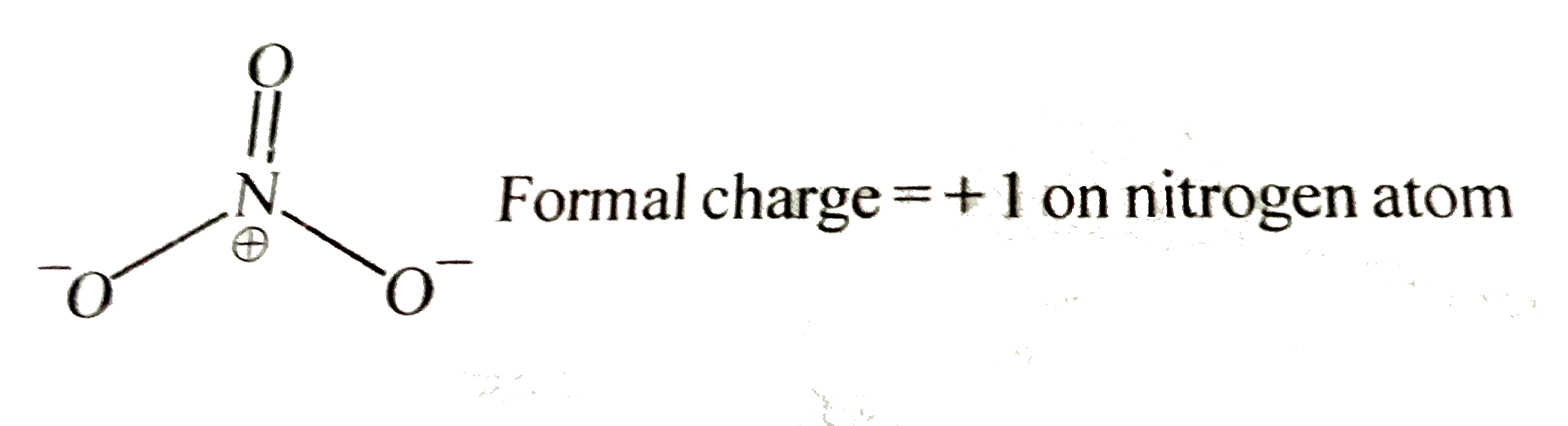 Formal charge = + 1 on nitrogen atom
Formal charge = + 1 on nitrogen atom