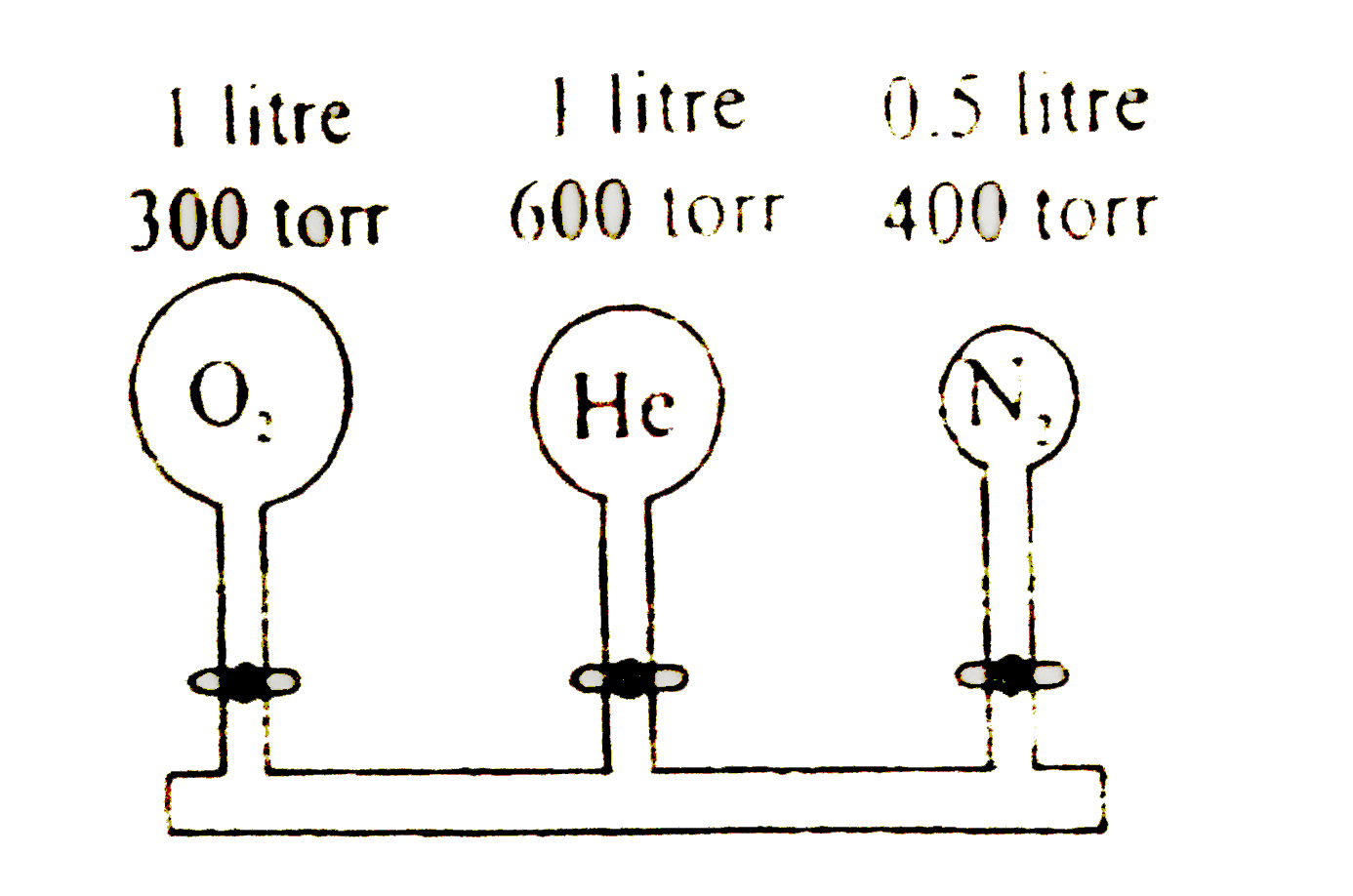A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- Identify the correct statement.
Text Solution
|
- If molality of pure gas A is (100)/(1.2) then calculate its molar mass...
Text Solution
|
- Consider the arrangement of bulbs shown in the drawing. Each of three ...
Text Solution
|
- How many photos at 620 nm must be absorbed to melt 10 gm of ice. If 32...
Text Solution
|
- If degree of dissociation of HI is 0.1 then K(P) for reaction is: 2H...
Text Solution
|
- Identify the correct statement for SO(3) (g) hArr SO(2) (g) + (1)/(2...
Text Solution
|
- Calculate the de-Brogle wavelength of an electron whose kinetic energy...
Text Solution
|
- What is the shortest wavelength in Bracket series of He^(+) spectrum ?
Text Solution
|
- Indicate the correct order of acidic strength (first ionization ) in t...
Text Solution
|
- Consider the following reaction : 8H(2) (g) + S(8) (l) rarr 8H(2) S ...
Text Solution
|
- Which of the following statement is correct ?
Text Solution
|
- What volume of aqueous solution of NaOH that is 80% by mass NaOH, cont...
Text Solution
|
- Which of the following is correct order of -1 ?
Text Solution
|
- Ammonium bydrogen sulfied, NH(4)HS is unstable at room temperature and...
Text Solution
|
- Which of the following pairs of structures does not represent valid re...
Text Solution
|
- SO(3) (g) is produced as : SO(2) (g) + (1)/(2) O(2) (g) hArr SO(3) (...
Text Solution
|
- Which compound has identical C-C bond length.
Text Solution
|
- Wavelength of an oxgyen molecule, O(2), travelling at 500 m//sec is :
Text Solution
|
- Which mentioned bond has highest C-H bond dissociation energy.
Text Solution
|
- Identify the correct statement regarding Vangerwaal gas.
Text Solution
|