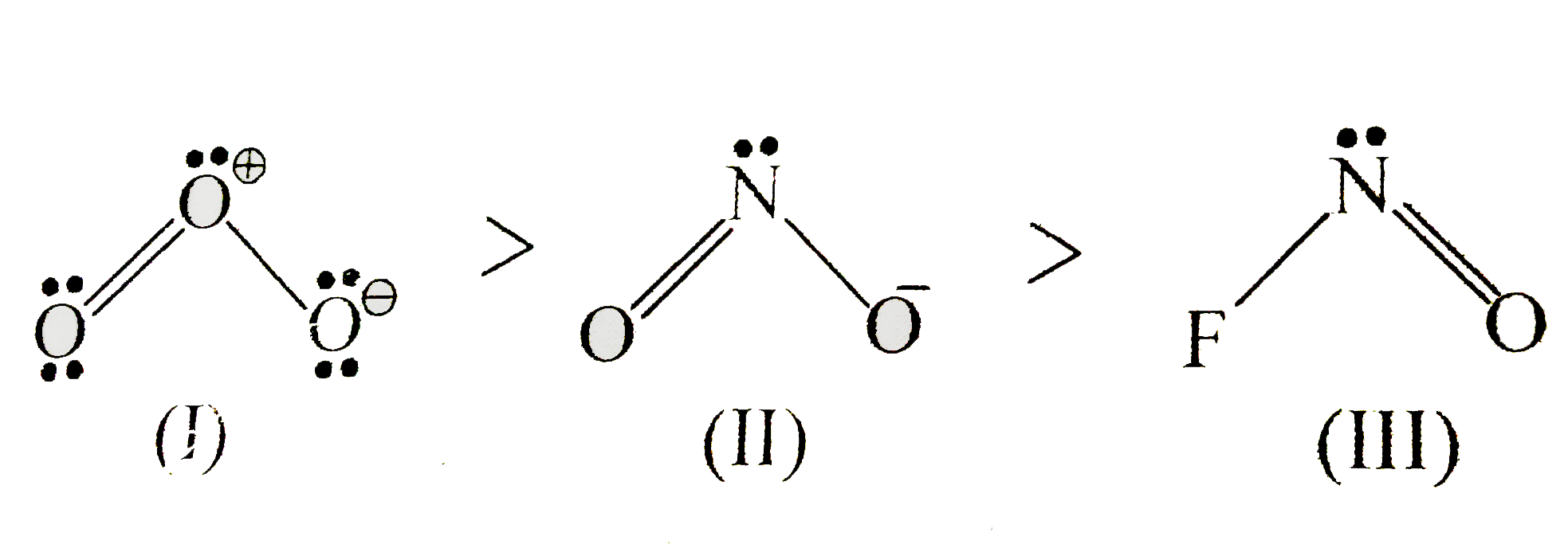
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- A student Babita Phogat went to meet his friend Geeta Phogat, where sh...
Text Solution
|
- Which resonating struction is most stable ?
Text Solution
|
- Arrange the following speices according to their bond angle order. (...
Text Solution
|
- Which of the following is strongest base ?
Text Solution
|
- Which of the following species is/are isoelectronic to each other ? ...
Text Solution
|
- Degree of unsaturation for
Text Solution
|
- The species which is not tetrahedral in shape is
Text Solution
|
- Which of the following is least basic
Text Solution
|
- Which of the order is incorrect ?
Text Solution
|
- Which of the following set of Quantum numbers is not possible?
Text Solution
|
- Which of the following has maximum unpaired electrons ?
Text Solution
|
- The correct option regarding size of orbitals is:
Text Solution
|
- The correct set of possible quantum number number (n,1,m,s,) for las...
Text Solution
|
- Select the correct statement(s).
Text Solution
|
- Which of the following energy level can not exist according to quantum...
Text Solution
|
- Calculate 'Q' for last electron of Ga. where Q=n+l+ maximum possible...
Text Solution
|
- Calculate total number of orbitals having (n+l) value=8 and magnetic q...
Text Solution
|
- How many orbitals, contains at least one electron in the ground state ...
Text Solution
|
- Calculate the total number of p-orbitals electrons present in Cu(29) a...
Text Solution
|
- Final the total number of species having two unparired electron from t...
Text Solution
|