Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILL BASED QUESTIONS|4 VideosPERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise Test Your Knowledge (Very Short Answer Questions)|15 VideosPERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise Short Ansewer Questions|41 VideosMETALS AND NON-METALS
DINESH PUBLICATION|Exercise MCQ|64 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-Long Answer Questions
- The atomic radii of the elements of second period are given below: ...
Text Solution
|
- 'The atomic number of Cl is 17. On the basis of this information, answ...
Text Solution
|
- The list of the elements present in the same period but in different g...
Text Solution
|
- An element E has following electronic configuration : {:(K,L,M,),(2,...
Text Solution
|
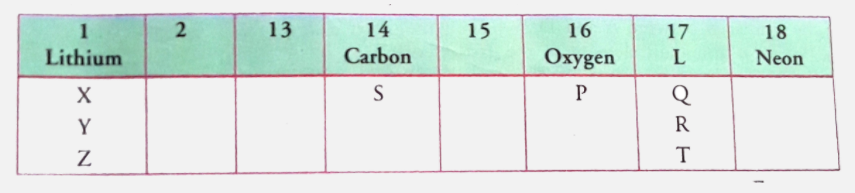
- From the part of the periodic table given, answer the following questi...
Text Solution
|
- Atomic number of an element is 16. Predict. (i) the number of valenc...
Text Solution
|
- (a) How are the following related ? (i) Number of valence electrons ...
Text Solution
|
- The position of some elements A, B, C, D, E, F, and G in the Modern Pe...
Text Solution
|
- (a) The modern periodic table has been evolved through the early ...
Text Solution
|
- An element is placed in 2^("nd") group and 3^("rd") period of the peri...
Text Solution
|
- An element X (atomic number 17) reacts with an element Y (atomic numbe...
Text Solution
|
- Atomic number of few elements are given below 10, 20, 7, 14 (a) Iden...
Text Solution
|
- (a) In this ladder (Figure) symbols of elements are jumbled up. Rearra...
Text Solution
|
- Complete the following crossword puzzle (Figure) Across (1) An ele...
Text Solution
|
- Mendeleev predicted the existence of certain elements not known at tha...
Text Solution
|
- (a) Electropositive nature of the element(s) increases down the group ...
Text Solution
|
- An element X which is a yellow solid at room temperature shows catenat...
Text Solution
|
- An element X of group 15 exists as diatomic molecule and combines with...
Text Solution
|
- Which group of elements could be placed in mendeleev's periodic table ...
Text Solution
|
- Give an account of the process adopted by Mendeleev for the classifica...
Text Solution
|