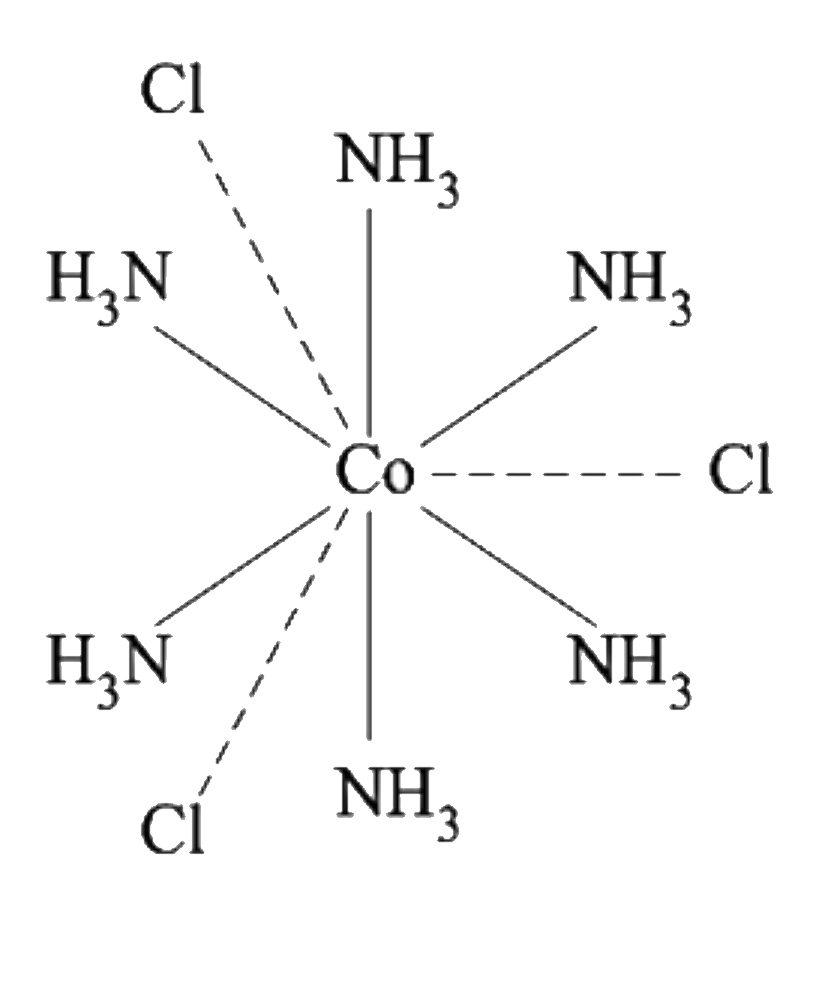
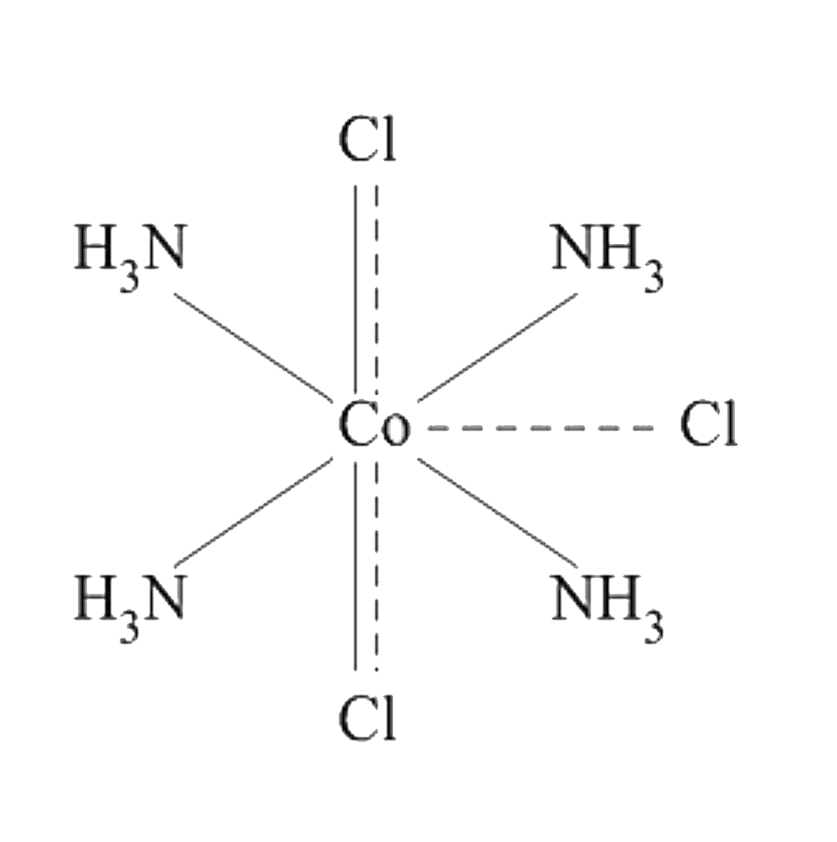
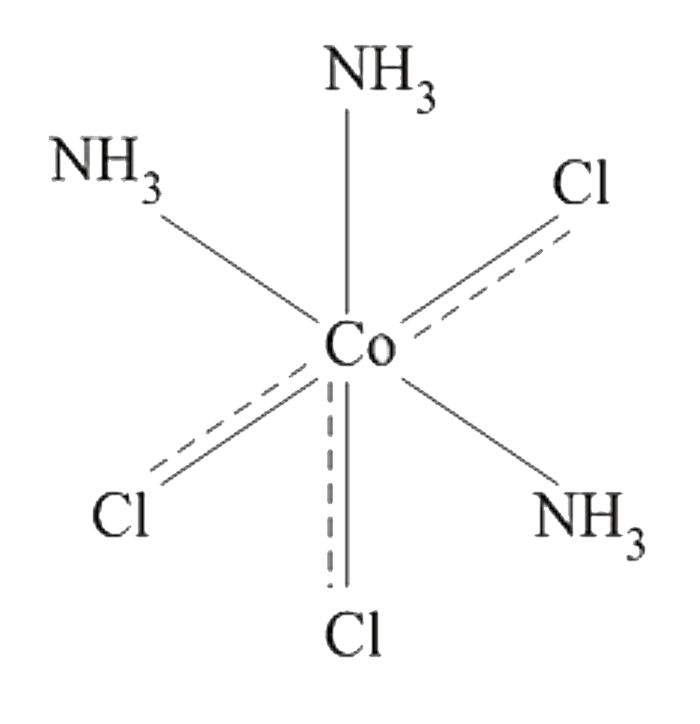
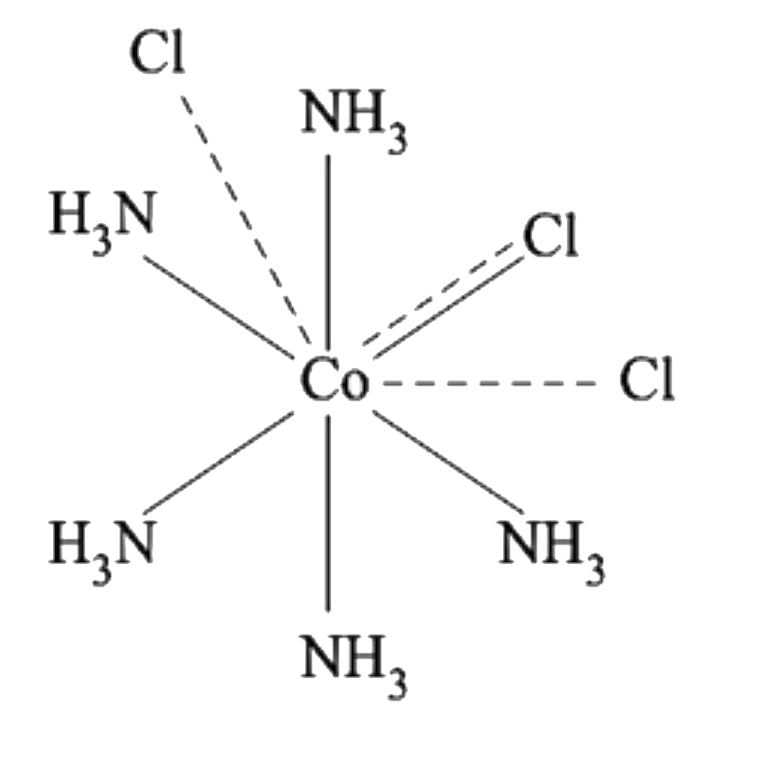
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 92-CHEMISTRY
- Which equilibrium can be described as an acid- base reaction using the...
Text Solution
|
- which of the following graph is correct representation between atomic ...
Text Solution
|
- The solution of which of the following will be non - conducting ?
Text Solution
|
- Product of the given reaction CH3CH2-CH=CH3overset(SiO2)rarr will be
Text Solution
|
- An endotthermic reaction is non-spontaneous at freezing point of water...
Text Solution
|
- 2Ag^(+)(aq) + Cu(s) rightarrow Cu^(2+)(aq) + 2Ag(s) The standard pot...
Text Solution
|
- A salt which on hearing with conc. H2 SO4 gives violet vapours is
Text Solution
|
- Which of the following electronic configurations have spin multiplicit...
Text Solution
|
- underset(("Coloured solution"))(P)+BaCl(2) to underset(("White"))(Qdar...
Text Solution
|
- Which of the following plots represents the behavior of an ideal binar...
Text Solution
|
- Arrange these compounds in decreasing order of reactivity for the nucl...
Text Solution
|
- In the given reaction CH3-CH2-COOHoverset((i)AgNO3)underset((ii)Br2//D...
Text Solution
|
- For vaporization of water at 1 atmospheric pressure, the values of Del...
Text Solution
|
- Which among the given compounds will give thermal elimination ?
Text Solution
|
- In the electrolysis of silver nitrate, the mass of silver deposited is...
Text Solution
|
- When NaCl is added to the reaction mixture of an oil and caustic soda...
Text Solution
|
- Reactivity of HCHO with the following Grignard reagent in the decreasi...
Text Solution
|
- In Purine nucleosides C - 1 of sugar forms glycosidic linkage with whi...
Text Solution
|
- Below critical micelle concentration (CMC):
Text Solution
|
- The reaction , N2O4(g)rarr2NO2(g) , is first order reaction , which of...
Text Solution
|