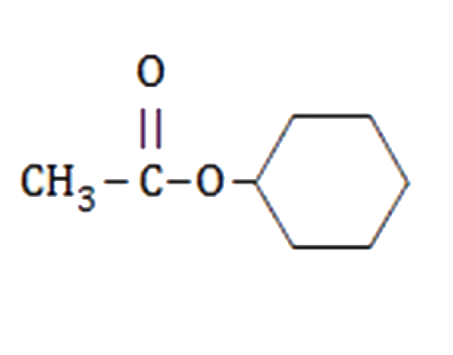
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 93-CHEMISTRY
- The equilibrium constant for the decomposition of water H2O(g) hArr H2...
Text Solution
|
- A 50 ml solution of pH=1 is mixed with a 50 ml solution of pH=2. The p...
Text Solution
|
- Which of the following is most reactive for hydrolysis ?
Text Solution
|
- The aqueous solution containing one mole of CoCl(3).5NH3 consumed 2 mo...
Text Solution
|
- Which alkene will give optically inactive product with Br2//"CCl"4 ?
Text Solution
|
- The difference between the heats of reaction at constant pressure and ...
Text Solution
|
- When aluminium oxide (Al(2)O(3)) is electrolysed for the production of...
Text Solution
|
- Incorrect match for give complex compound/ion and its characteristics
Text Solution
|
- Wave function of an orbital is plotted against the distance from nucle...
Text Solution
|
- When a large amount of KMnO4 is added to concentrated , H2SO4 an explo...
Text Solution
|
- A binary solution contains x1 and x2 mile fraction of two components h...
Text Solution
|
- In the reaction CH3-CH2-OHunderset((ii)LiAiH4)overset((i)TsCl)rarr(X) ...
Text Solution
|
- Which of the following statement are correct ? (1) alpha - amino aci...
Text Solution
|
- Which of the following statement is wrong ?
Text Solution
|
- Salt used for performing bead test in qualitative inorganic analysis i...
Text Solution
|
- Zn+Cu^(2+)(aq)toCu+Zn^(2+)(aq). Reaction quotient is Q=([Zn^(2+)])/(...
Text Solution
|
- Which of the following anions is the weakest base ?
Text Solution
|
- The weight of ethyl alcohol which must be added to 1.0 L of water so t...
Text Solution
|
- Which one of the following nitroalkanes will give nitrolic acid with N...
Text Solution
|
- The coagulation value in millimoles per litre of electrolytes used for...
Text Solution
|