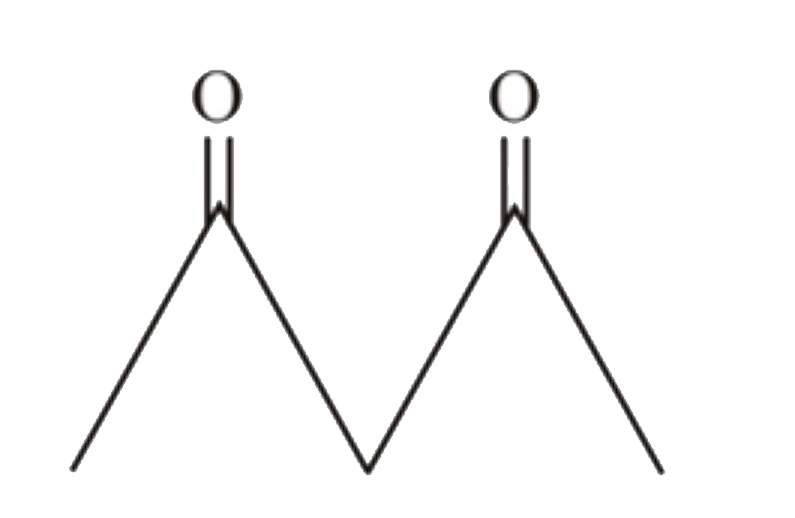
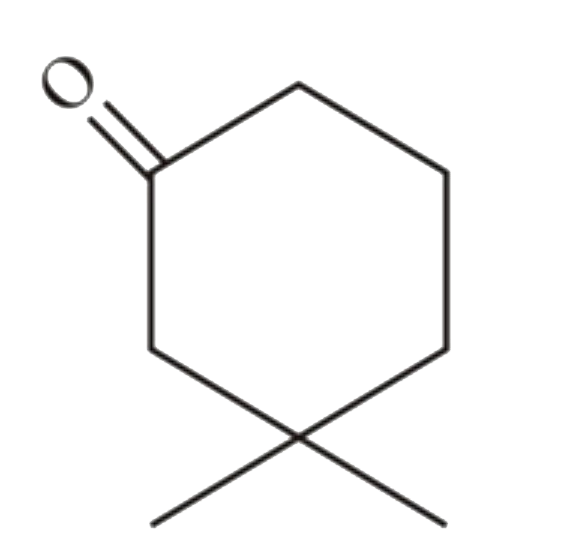
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 95-CHEMISTRY
- Consider the following reaction CH3-CH=CH2overset(Br2//NaCl)rarr Produ...
Text Solution
|
- Which of the following combination does not evolve Cl(2) gas?
Text Solution
|
- Most acidic hydrogen is present in
Text Solution
|
- Consider the following reaction M+O2 rarrMO2 (M= alkali metal) (stable...
Text Solution
|
- Two isomeric compounds Cl-CH2-CH2-CH2Cl and CH3-CH2-CHCl2 can be dis...
Text Solution
|
- Which compound will liberate oxygen when reacts with ice cold water ?
Text Solution
|
- Which metal is protected by layer of its own oxide ?
Text Solution
|
- The pKb value of ammonium hydroxide is 4.75. An aqueous solution of am...
Text Solution
|
- In the given reaction [X] will be :
Text Solution
|
- Equilibrium constant for two complexes are A: K4[Fe(CN)6]2.6xx10^(37) ...
Text Solution
|
- the incorrect statement with respect to S(N^(1) " and " S(N^(2)) mech...
Text Solution
|
- Which of the following metals can be extracted by smelting ?
Text Solution
|
- A compound contains three elements A,B and C, if the oxidation number ...
Text Solution
|
- Which one of the following is an Z isomer ?
Text Solution
|
- Which of the following statement is correct ?
Text Solution
|
- Co-ordination number (CN) of barium ion (Ba^(2+)) in BaF^(2) is 8. Wha...
Text Solution
|
- If a 6.84%(wt,vol.)solutionof cane sugar(mol.Wt.=342)is isotonic with ...
Text Solution
|
- In the reaction X + Y underset(5^(@)C)overset(NaOH)(rarr) CH(3)-ov...
Text Solution
|
- In the reaction sequence CH3-CH2-COOHoverset(H2O2)rarr[X]overset(Delta...
Text Solution
|
- Volume of 0.1 M K2Cr2O7 required to oxidize 35 ml of 0.5 M FeSO4 solut...
Text Solution
|