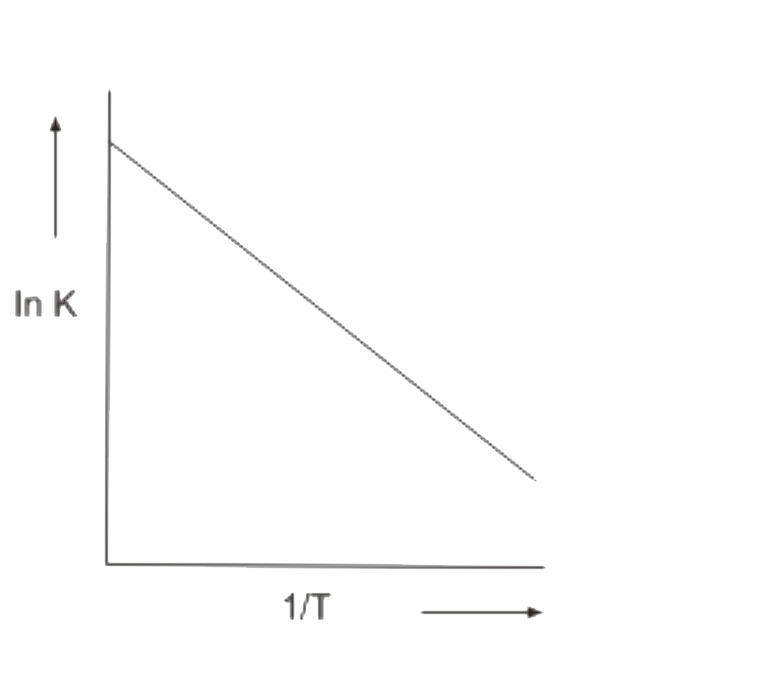
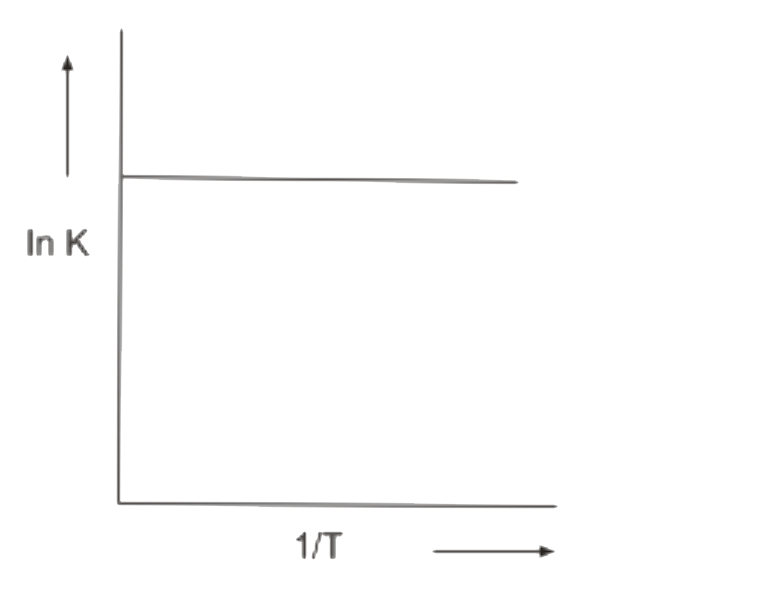
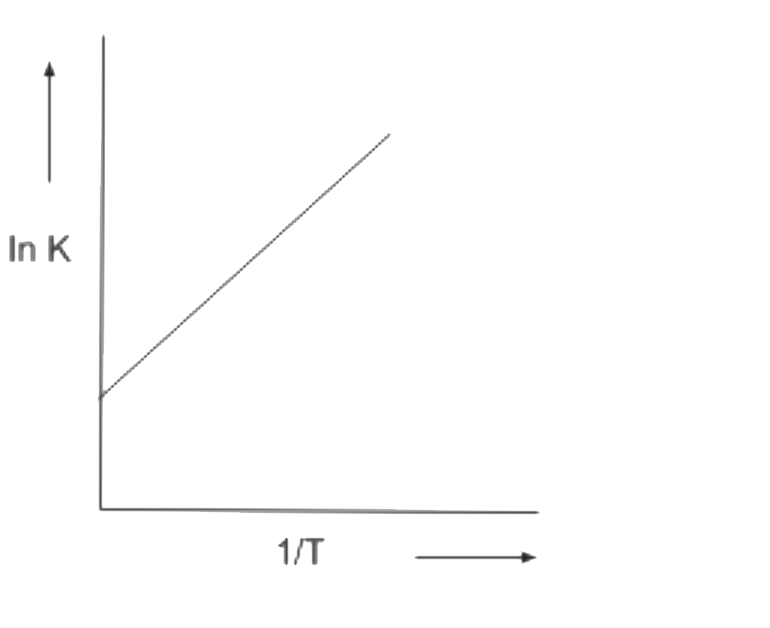
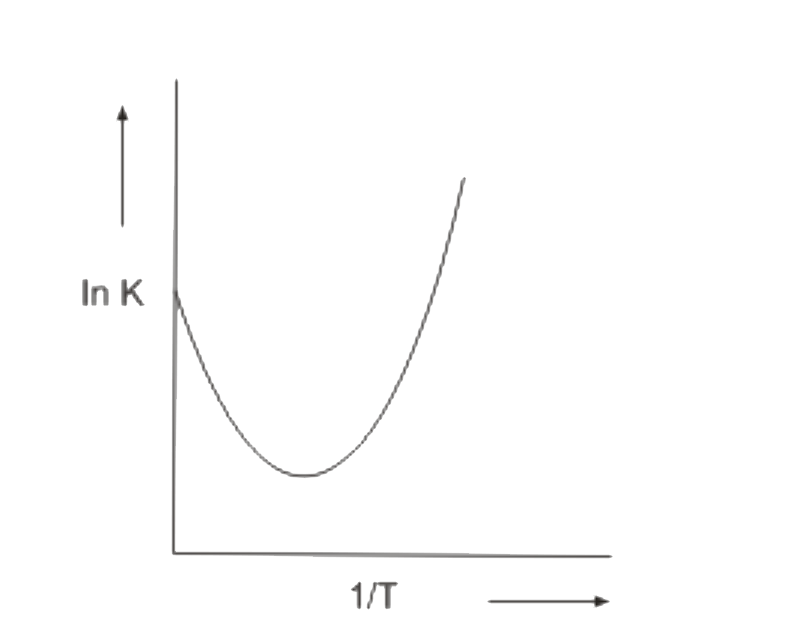
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 84-CHEMISTRY
- Alcohol (X)overset("aq. NaOH"+I(2))rarrCHI(3)+(Y)overset(H(3)O^(+))rar...
Text Solution
|
- An element crystallizes both in fcc and bcc lattice. If the density of...
Text Solution
|
- The CFSE for octahedral [CoCl(6)]^(4-) is 18,000 cm^(-1). The CFSE for...
Text Solution
|
- CH(3)COCl+H(2)underset("Quinoline")overset(Pd//BaSO(4))rarr
Text Solution
|
- The vapour pressure of water at T(K) is 20 mm Hg. The following soluti...
Text Solution
|
- XCl(2)("excess")+Ycl(2) to XCl(4)+Y darr , YO underset(gt400^(@))ove...
Text Solution
|
- Match list I with list II and select the correct answer using the code...
Text Solution
|
- Which among the following reactions is can be a example of pseudo firs...
Text Solution
|
- A chemical A is used for the preparation of washing soda to recover am...
Text Solution
|
- Pick out the correct statement.
Text Solution
|
- Which is not true about borax?
Text Solution
|
- (A)(C(4)H(8)O)overset(H(3)O^(o+))rarr(B)overset(CrO(3))underset("aceti...
Text Solution
|
- Which In K vs 1/T plot is correct for an equilibrium that shits toward...
Text Solution
|
- What is Z in the following sequence of reactions ? 2-methyl -2-bromo p...
Text Solution
|
- Which of the following salt on heating with solid K(2)Cr(2)O(7) and Co...
Text Solution
|
- In the reaction 8Al+3Fe(3)O(4)rarr 4Al(2)O(3)+9Fe the number of el...
Text Solution
|
- Total number of enol possible for the compound formed during given rea...
Text Solution
|
- Calculate pH at which an acid indicator Hin with concentration 0.1M ch...
Text Solution
|
- CH(3)-underset(Br)underset(|)overset(Br)overset(|)C-undersset(Br)under...
Text Solution
|
- Total number of covalent bonds in C(3)O(2) is x and y is the number of...
Text Solution
|