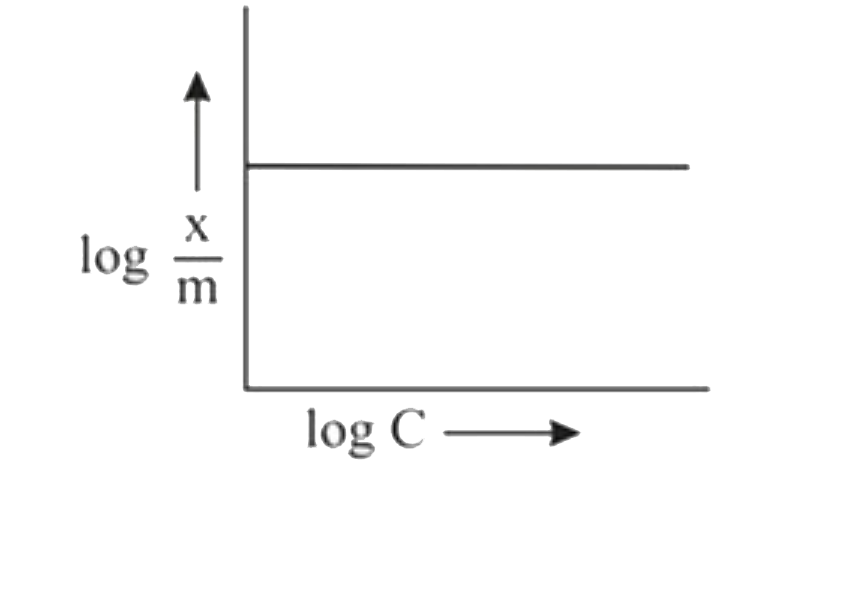
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 86-CHEMISTRY
- The dipole moment of HBr is 2.6xx10^(-30) esu.cm and the interatomic s...
Text Solution
|
- The simultaneous solubility of AgCN (K(sp)=2.5xx10^(-6))and AgCl (K(sp...
Text Solution
|
- The degree of adsorption of solution on solid surface depends on conce...
Text Solution
|
- The solubility of metal halides depends on their nature, Lattice entha...
Text Solution
|
- The addition of NaOH to Cr^(3+) solution produces the precipitate of
Text Solution
|
- MnO(4)^(-) is good oxidising agent in different medium changing to - ...
Text Solution
|
- Arrhenius equation k=Ae^(-E(a)//RT) If the activation energy of the re...
Text Solution
|
- Two complexes [Cr(H(2)O(6))(6)]Cl(3) and [Cr(NH(3))(6)]Cl(3) (B) are v...
Text Solution
|
- Match the refining methods (Column I) with metals (Column II)
Text Solution
|
- A solution is prepared containing a 2:1 mol ration of dibromoethane (C...
Text Solution
|
- Which of the following expression is correct for packing fraction of N...
Text Solution
|
- Consider the following compounds Order of basicity of these compo...
Text Solution
|
- Which of the following functional groups is generally least reactive t...
Text Solution
|
- In the given reaction CH(3)-cH(2)COOHoverset((i)CH(3)Li" (excess)"(ii)...
Text Solution
|
- Which one of the following will not give alkyl cyanide on treatment wi...
Text Solution
|
- Pick out the incorrect statement
Text Solution
|
- In the given reaction CH(2)=CH-CHOoverset([X])rarrCH(2)=CH-CH(2)OH ...
Text Solution
|
- Ammonium perchlorate, NH4ClO4 , used in the solid fuel in the booster ...
Text Solution
|
- The haemoglobin from the red blood corpuscles of most mammals contains...
Text Solution
|
- For the equilibrium AB(g) hArr A(g)+B(g) at a given temperature, the p...
Text Solution
|