Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 93-CHEMISTRY
- Consider the given compounds : (a) CH(3)-CH(2)-NH(2) (b) CH(3)-C...
Text Solution
|
- Which of the following ionic/molecular species does not disproportiona...
Text Solution
|
- In correct statement regarding following reaction is
Text Solution
|
- In the given reaction [X] will be
Text Solution
|
- An ideal gas is taken around the cycle ABCA as
Text Solution
|
- CuSO(4)(aq)overset(H(2)Suarr)rarrMdarroverset("Excess of KCN")rarrN+O ...
Text Solution
|
- In the reaction sequence will be
Text Solution
|
- Equilibrium constant K(C) for the following reaction at 800 K is, 4 NH...
Text Solution
|
- Arrange the following cyano complexes in decreasing order of their mag...
Text Solution
|
- A reactant (A) forms two products A overset (k(1))rarr B, Activation...
Text Solution
|
- Pyroxenes are class of silicate minerals, which exhibit a polymeric ch...
Text Solution
|
- In the given reaction [X] will be
Text Solution
|
- Mg(s)|Mg^(2+)(aq)||Zn^(2+)(aq)|Zn(s),E^(@)=+3.13V The correct plot o...
Text Solution
|
- In the reaction sequence C(6)H(5)CHO overset(NH(2)OH//H^(o+))underse...
Text Solution
|
- Choose the correct sequence for the geometry of the given molecules ...
Text Solution
|
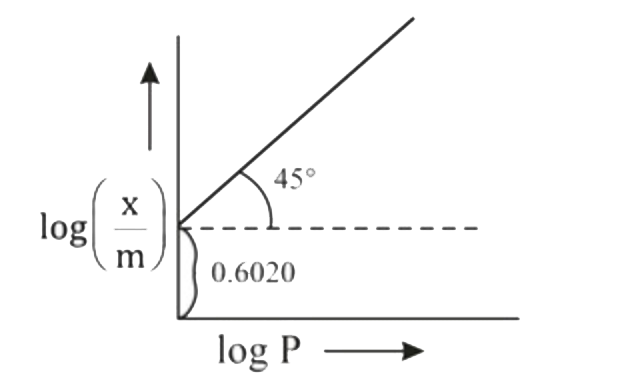
- Graph between log((x)/(m)) and log P is straight line at angle of 45^(...
Text Solution
|
- Find the sum of bond order between same bonded atoms in Q and R compou...
Text Solution
|
- How many mL of 22.4 volume H(2)O(2) is required to oxidise 0.1 mol of ...
Text Solution
|
- K(a) for HCN is 5xx10^(-10) at 25^(@)C. For maintaining a constant pH ...
Text Solution
|
- How many -OH groups are present in one molecules of sucrose?
Text Solution
|