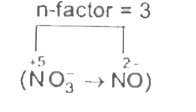
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise SINGLE INTEGER TYPE QUESTIONS|2 VideosSTOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise MATRIX - MATCH TYPE|2 VideosSTOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise SOLVED PROBLEMS (SUBJECTIVE )|16 VideosSOLID STATE
FIITJEE|Exercise (SINGLE INTEGER ANSWER TYPE QUESTION)|5 VideosTEST PAPERS
FIITJEE|Exercise CHEMISTRY|747 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-STOICHIOMETRY AND BALANCING REDOX REACTION -SOLVED PROBLEMS (OBJECTIVE )
- The amount of hydrazine (N(2)H(4)) oxidized to N(2) by 19.4 g K(2)Cr...
Text Solution
|
- Magnetite, Fe(3)O(4), can be converted into metallic iron by heating w...
Text Solution
|
- In basic medium , CrO(4)^(2-) oxidize S(2)O(3)^(2-) to form SO(4)^(2-...
Text Solution
|
- 20mL of 0.2M Al(2)(SO(4))(3) is mixed with 20mL of 0.6M Bacl(2).Conce...
Text Solution
|
- The weight of sodium bromate required to prepare 85.5 ml " of " 0.67...
Text Solution
|
- NalO(3) reacts with NaHSO(3) according to equation lO(3)^(-) + 3HSO...
Text Solution
|
- If 0.5 mole of BaCl(2) is mixed with 0.20 mole of Na(3)PO(4), the maxi...
Text Solution
|
- 34 g of hydrogen peroxide is present in 1120 ml of Solution. This solu...
Text Solution
|
- The number of moles of KMnO(4) that will be needed to react completely...
Text Solution
|
- What volume of 0.1 M KMnO(4) is needed to oxidize 100 mg of FeC(2)O...
Text Solution
|
- What volume of 3 molar HNO(3) is needed to oxidise 8 g of Fe^(3+), HNO...
Text Solution
|
- 0.5 g of fuming sulphuric acid (H2SO4+SO3), called oleum, is diluted w...
Text Solution
|
- The minimum quantity of H(2)S needed to precipitate 64.5 g of Cu^(2+) ...
Text Solution
|
- You are provided with 1 M solution of NaNO(3) whose density = 1.25 g/...
Text Solution
|
- Which of the following is are redox reaction(s) ?
Text Solution
|
- In the reaction 3Br(2) + 6CO(3)^(2-) + 3H(2)O to 5Br^(-) + 2BrO(3)^(...
Text Solution
|
- 0.5 g of metal nitrate gave 0.43 g of metal sulphate
Text Solution
|
- 25 ml of H(2)O(2) solution was added to the excess of acidified Kl sol...
Text Solution
|
- Percentage strength of the above H(2)O(2) solution is :
Text Solution
|
- The volume strength of the H(2)O(2) solution is :
Text Solution
|