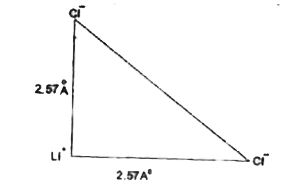
Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE)|25 VideosSOLID STATE
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE) REASONING TYPE QUESTIONS|1 VideosSOLID STATE
FIITJEE|Exercise (SINGLE INTEGER ANSWER TYPE QUESTION)|5 VideosQUALITATIVE ANALYSIS
FIITJEE|Exercise Single interger answer type|3 VideosSTOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-SOLID STATE-SOLVED PROBLEMS (SUBJECTIVE)
- Metallic gold crystallizes in fcc lattice. The length of the cubic un...
Text Solution
|
- Ferrous oxide (FeO) crystal has a cubic structure and each edge of the...
Text Solution
|
- The composition of a sample of wustite is Fe(0.93)O(1.00) What percent...
Text Solution
|
- Potassium crystallizes in body centered cubic lattice with a unit cell...
Text Solution
|
- A compount AB has a rock type structure with A:B=1:1. The formula weig...
Text Solution
|
- An element crystallises as face-centred cubic lattice with density as ...
Text Solution
|
- Calcium crystallizes in a face centred cubic unit cell with a = 0.556 ...
Text Solution
|
- Radii of A^(+) and that of X^(-) and Y^(-) have been given as A^(+...
Text Solution
|
- The unit cell cube length for LiCl(just like NaCl structures) is 5.14Å...
Text Solution
|
- The density of potassium bromide crystal is 2.75 g cm^(-3) and the len...
Text Solution
|
- When an electron in an excited state of Mo atoms falls from L to K-she...
Text Solution
|
- The unit cell length of NaCl is observed to be 0.5627 nm by X-ray diff...
Text Solution
|
- The density of CaO is 3.35xx10^(3)kg*m^(3). This oxide crystallises in...
Text Solution
|
- Copper has fcc crystal structure. Assuming an atomic radius of 130 pm ...
Text Solution
|
- A metal crystallizes into two cubic phases, face-centred cubic and bod...
Text Solution
|
- In a crystalline solid, atom of A form fcc packing and atoms of B occu...
Text Solution
|
- In face centred cubic (fcc) crystal lattice edge length of the unit ce...
Text Solution
|