A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
IONIC EQUILIBRIUM
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE ) LEVEL - I REASONING TYPE QUESTIONS|4 VideosIONIC EQUILIBRIUM
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE ) LEVEL - II|20 VideosIONIC EQUILIBRIUM
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE ) LEVEL -II|13 VideosHYDROCARBONS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTION|9 VideosLIQUID SOLUTION
FIITJEE|Exercise Single Integer Answer Type Question|10 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-IONIC EQUILIBRIUM -ASSIGNMENT PROBLEMS (OBJECTIVE ) LEVEL - I
- At a certain temperature , K(w) of water is 9.0 xx 10^(-14) . The pH...
Text Solution
|
- Which of the following will have nearly equal [H^(+)] concentration...
Text Solution
|
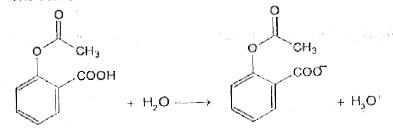
- The active ingredients in aspirin is acetly salicylic acid wit...
Text Solution
|
- The pair of compounds, which cannot exist together in a solution is-
Text Solution
|
- Equal volume of 0.02 M KCl and 0.02 M K[Ag(CN)(2)] are mixed , then...
Text Solution
|
- A certain weak acid has a dissocation constant of 1.0 xx 10^(-4). The ...
Text Solution
|
- From separate solutions of sodium salts, NaW, NaX, NaY and NaZ have pH...
Text Solution
|
- The pK(a) of acteylsalicylic acid (aspirin) is 3.5. The pH of gastric ...
Text Solution
|
- The ionization constant of overset(o+)(NH(4)) ion in water is 5.6 xx 1...
Text Solution
|
- In the equilibrium CaF(2) hArr Ca^(2+) +2F^(-) , the (Ca^(2+)) is i...
Text Solution
|
- 10 ml of 0.2 M acid is added to 250 ml of a buffer solution with p...
Text Solution
|
- 20g of a binary electrolyte(mol.wt.=100)are dissolved in 500g of water...
Text Solution
|
- The solubility of CsBr(3) (MW = 373) in water is 746 ppm . The solu...
Text Solution
|
- The precipitate of CaF(2) (K(sp)=1.7xx10^(-10)) is obtained when equal...
Text Solution
|
- A weak monobasic (0.1 M) has a pH of 3 at a particular tempertaure ...
Text Solution
|
- The pH of 1 M PO(4)^(3-) (aq) solution (given pK(b) of PO(4)^(3-) =...
Text Solution
|
- pH of a saturated solution of M(OH)(2) is 13 . Hence K(sp) of M(OH)(2...
Text Solution
|
- A solution is a mixture of 0.05 M NaCl and 0.05 M AgI. The concentrati...
Text Solution
|
- For a 'C'M concentarted solution of a weak electrolyte A(x)B(y)alpha(d...
Text Solution
|
- Let the solubilities of AgCI in H(2)O, and in 0.01M CaCI(2), 0.01M NaC...
Text Solution
|