Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
FIITJEE|Exercise Solved Problems (Objective)|41 VideosELECTROCHEMISTRY
FIITJEE|Exercise Matrix -Match Type|1 VideosELECTROCHEMISTRY
FIITJEE|Exercise Matcing Type Single Correct Questions|3 VideosCONCEPT OF ACIDS AND BASES
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|4 VideosELECTROPHILIC AROMATIC SUBSTITUTION
FIITJEE|Exercise NUMERICAL BASED QUESTIONS INDICATED BY DIGITAL INTERGER|1 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-ELECTROCHEMISTRY-Solved Problems (Subjective)
- Calculate the emf of the cell. Mg(s)|Mg^(2+) (0.2 M)||Ag^(+) (1xx10^...
Text Solution
|
- The standard reduction potential for Cu^(2+)//Cu is +0.34V. Calculate ...
Text Solution
|
- A metal is known to form fluoride MF(2). When 10 ampere electricity is...
Text Solution
|
- Calculate the potential of an indicator electrode versus the standard ...
Text Solution
|
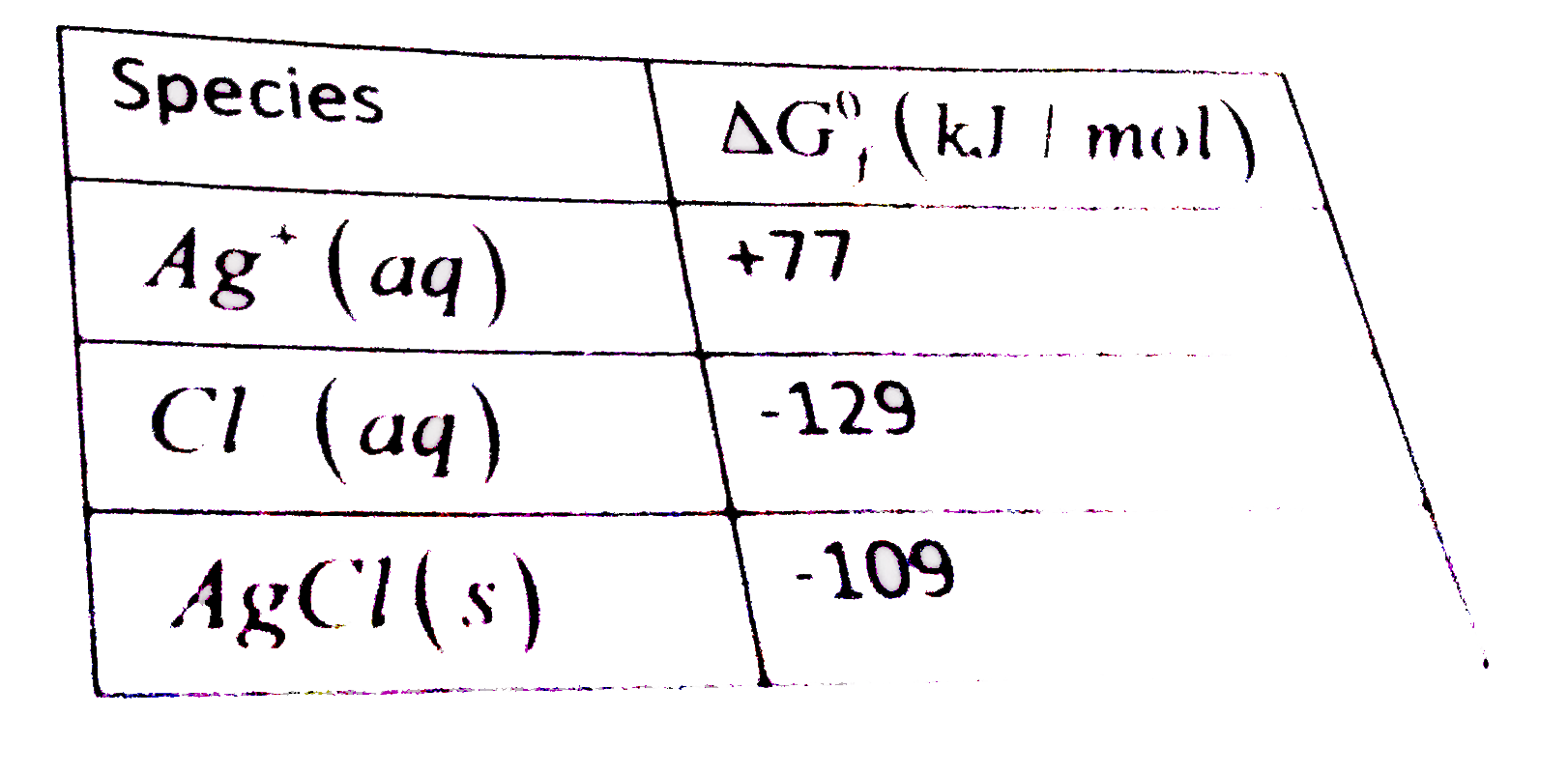
- a. For the reaction Given : Ag^(o+)(aq)+Cl^(o+)(aq)rarr AgCl(s) ...
Text Solution
|
- Chromium metal can be plated out from an acidic solution containing Cr...
Text Solution
|
- During the discharge of a lead storage battery, the density of sulphur...
Text Solution
|
- In an electrolysis experiment, current was passed for 5h through two c...
Text Solution
|
- At 25^(@)C the equivalent conductance of butanoic acid at infinite dil...
Text Solution
|
- The following galvanic cell was underset(100mL) (Zn|Zn^(2+) (1M) )||...
Text Solution
|
- The overall formation constant of the following reaction is 1 xx 10^(1...
Text Solution
|
- Calculate the minimum mass of NaOH required to be added in RHS to cons...
Text Solution
|
- For the cell Mg((s))|Mg((aq))^(++)||Ag((aq))^(+)|Ag((s)) Calculate t...
Text Solution
|
- Estimate the cell potential of a Daniel cell having 1.0Zn^(++) and ori...
Text Solution
|
- If [Zn^(2+)] is maintained at 1.0 M (i) What is the minimum (Cu^(2+)...
Text Solution
|
- An aqueous solution of NaCl on electrolysis gives H(2(g))Cl(2(g)) and ...
Text Solution
|
- 1 litre aqueous solution of NaCl was electrolysed bètweern Pt electrod...
Text Solution
|
- Copper sulphate solution (250 ML) was electrolyzed using a platinum an...
Text Solution
|
- The following electrochemical cell has been set up. Pt((I))|Fe^(3+),...
Text Solution
|
- A constant current was flown for 2 hour through a solution of KI At th...
Text Solution
|