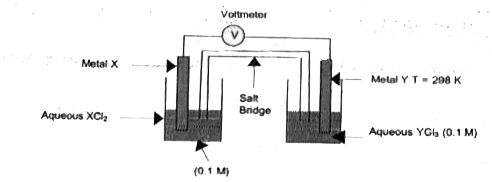
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
FIITJEE|Exercise Matrix Match Type Questions|8 VideosELECTROCHEMISTRY
FIITJEE|Exercise Matcing Type Single Correct Questions|3 VideosELECTROCHEMISTRY
FIITJEE|Exercise (Comprehension -IV)|4 VideosCONCEPT OF ACIDS AND BASES
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|4 VideosELECTROPHILIC AROMATIC SUBSTITUTION
FIITJEE|Exercise NUMERICAL BASED QUESTIONS INDICATED BY DIGITAL INTERGER|1 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-ELECTROCHEMISTRY-(Comprehension -V)
- Cell voltages and half-cell potentials are often compeared under stand...
Text Solution
|
- Cell voltages and half-cell potentials are often compeared under stand...
Text Solution
|
- Cell voltages and half-cell potentials are often compeared under stand...
Text Solution
|
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|