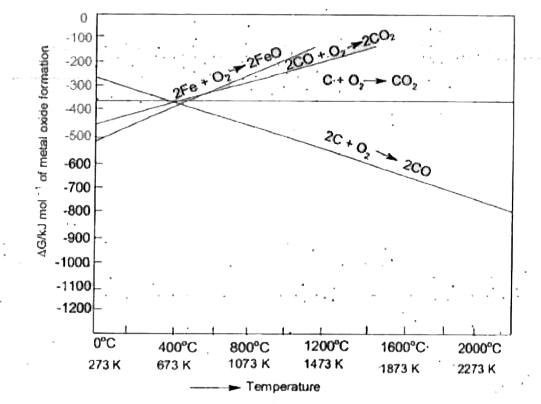
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORES AND METALLURGY
FIITJEE|Exercise MATRIX-MATCH THE FOLLOWING QUESTIONS|3 VideosORES AND METALLURGY
FIITJEE|Exercise MATCHING LIST TYPE QUESTIONS|3 VideosORES AND METALLURGY
FIITJEE|Exercise COMPREHENSION - II|2 VideosNUCLEIC ACID AND VITAMIN
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE)|15 VideosPERIODIC PROTERTIES OF ELEMENTS
FIITJEE|Exercise COMPREHENSION|13 Videos
Similar Questions
Explore conceptually related problems