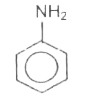
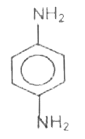
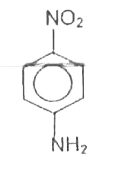
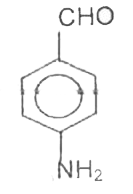
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CONCEPT OF ACIDS AND BASES
FIITJEE|Exercise ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-I REASONING TYPE QUESTONS|2 VideosCONCEPT OF ACIDS AND BASES
FIITJEE|Exercise ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-II|15 VideosCONCEPT OF ACIDS AND BASES
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE)Level-II|9 VideosCHEMISTRY IN EVERY DAY LIFE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II (Numerical Based Questions)|5 VideosELECTROCHEMISTRY
FIITJEE|Exercise Matcing Type Single Correct Questions|3 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-CONCEPT OF ACIDS AND BASES-ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-I
- What is the decreasing order of strength of bases?
Text Solution
|
- Which of the following is a soft base?
Text Solution
|
- Amongst the following the most basic compound is
Text Solution
|
- Which of the following is strongest Lewis base?
Text Solution
|
- The following are acid-base conjugate pairs except:
Text Solution
|
- The conjugate acid of S(2)O(8)^(-2)
Text Solution
|
- The following acids have been arranged in order of decreasing acid str...
Text Solution
|
- Which of the following are not amphiprotic in nature?
Text Solution
|
- Among the following compounds, the strongest acid is
Text Solution
|
- With reference to protonic acid which of the following statement is co...
Text Solution
|
- Barium peroxide when dissolved in water forms a substance which has ox...
Text Solution
|
- The following species cannot exist in an aqueous solution?
Text Solution
|
- Which of the following is the soft base?
Text Solution
|
- Which among the following is the strongest base?
Text Solution
|
- The conjugate base of [Al(H(2)O)(3) (OH)(3) is
Text Solution
|
- In the equilibrium CH(3)COOH + HFleftrightarrow CH(3)COOH(2)^(+) + F^(...
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Which of the following acids are likely to form Zwitter ions?
Text Solution
|
- Which of the following species is an acid and also a conjugate base of...
Text Solution
|
- The correct order of acidic strength is:
Text Solution
|