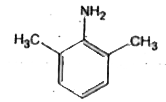
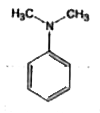
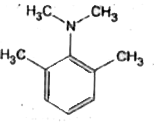
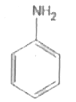
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CONCEPT OF ACIDS AND BASES
FIITJEE|Exercise ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-I REASONING TYPE QUESTONS|2 VideosCONCEPT OF ACIDS AND BASES
FIITJEE|Exercise ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-II|15 VideosCONCEPT OF ACIDS AND BASES
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE)Level-II|9 VideosCHEMISTRY IN EVERY DAY LIFE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II (Numerical Based Questions)|5 VideosELECTROCHEMISTRY
FIITJEE|Exercise Matcing Type Single Correct Questions|3 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-CONCEPT OF ACIDS AND BASES-ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-I
- Which of the following oxides of chromium is amphoteric in nature?
Text Solution
|
- In which of the following reaction does NH(3) acts as an acid?
Text Solution
|
- Find the correct order of acid strength of the following acids in aque...
Text Solution
|
- What is the basic strength order of the marked (a, b and c) nitrogen i...
Text Solution
|
- Which of the following compound possesses Lewis acid character? (1) B...
Text Solution
|
- Among the following pairs, the one representing a conjugate pair of an...
Text Solution
|
- The order of basic character of
Text Solution
|
- Arrange the following oxides in decreasing acidic order SiO(2) (I) ...
Text Solution
|
- The acid which forms two series of salt is
Text Solution
|
- Chose the correct acidity orders among the following. (1) H(3)AsO(4) ...
Text Solution
|
- If concentrations of two acids are same, their relative strengths can ...
Text Solution
|
- Which of the following is the strongest base?
Text Solution
|
- Identify the correct order of acidic strengths - CÓ(2), CuO, CaO, H(...
Text Solution
|
- The correct order of acidic strength is
Text Solution
|
- Which of the following is the correct order of basic nature of the com...
Text Solution
|
- Ammonium dichromate is used in some fire works. The green coloured pow...
Text Solution
|
- The correct order of basic strength is
Text Solution
|
- Conjugate acid of [Zn(OH)(H(2)O)(5)]^(2+) is
Text Solution
|
- The compound which has most acidic hydrogen
Text Solution
|
- Write the following in increasing order of pKa value
Text Solution
|