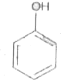
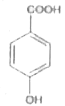
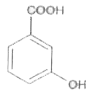
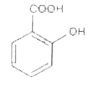
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CONCEPT OF ACIDS AND BASES
FIITJEE|Exercise COMPREHENSION-II|3 VideosCONCEPT OF ACIDS AND BASES
FIITJEE|Exercise COMPREHENSION-III|2 VideosCONCEPT OF ACIDS AND BASES
FIITJEE|Exercise ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-II|15 VideosCHEMISTRY IN EVERY DAY LIFE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II (Numerical Based Questions)|5 VideosELECTROCHEMISTRY
FIITJEE|Exercise Matcing Type Single Correct Questions|3 Videos
Similar Questions
Explore conceptually related problems