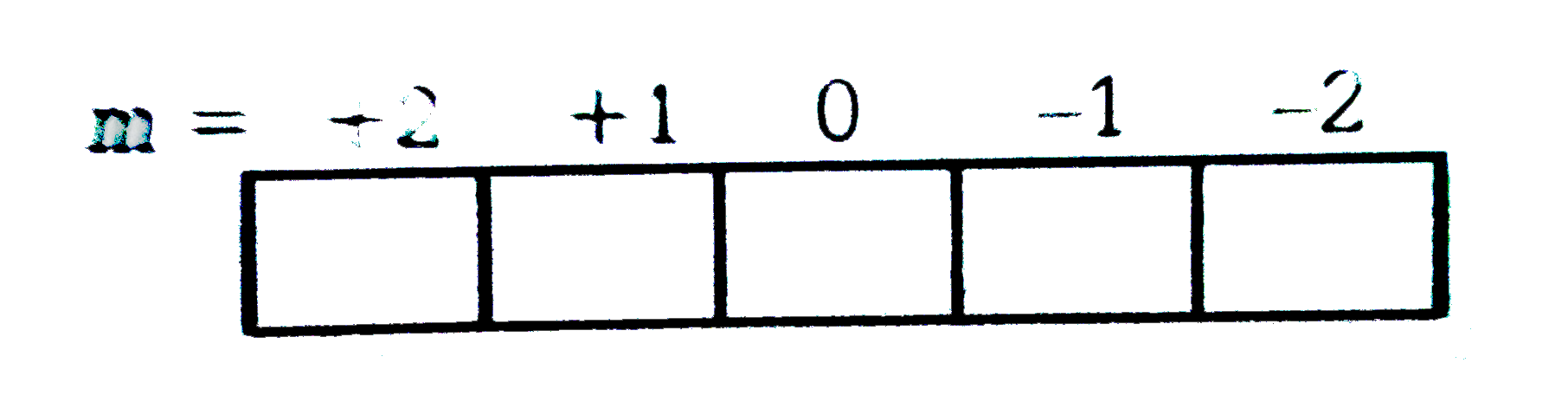A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-ATOMIC STRUCTURE-MCQ
- The value of Planck's constant is 6.63 xx 10^(-34)Js. The velocity of ...
Text Solution
|
- In hydrogen atom, energy of first excited state is - 3.4 eV. Then, KE...
Text Solution
|
- The following quantum numbers are possible for how many orbitals (s) n...
Text Solution
|
- The energy of a photon is given as, Delta E/atom = 3.03 xx 10^(-19)J "...
Text Solution
|
- The de-Broglie wavelength of a particle with mass 1 g and velocity 100...
Text Solution
|
- Who modified Bohr's theory of introducing elliptical orbits for electr...
Text Solution
|
- Which of the following electron configurations is correct for iron,(at...
Text Solution
|
- The uncertainty in momentum of an electron is 1 xx 10^-5 kg - m//s. Th...
Text Solution
|
- The position of both an electron and a helium atom is known within 1.0...
Text Solution
|
- The radus of hydrogen atom in the ground state 0.53 Å. The radius of L...
Text Solution
|
- The electronic configuration of gadolinium (Atomic number 64) is
Text Solution
|
- The momentum of a particle having a de-Broglie wavelength of 10^(17...
Text Solution
|
- The orbitals are called degenerate when
Text Solution
|
- The uncertainty in the position of an electron (mass = 9.1 xx 10^-28 g...
Text Solution
|
- The radus of hydrogen atom in the ground state 0.53 Å. The radius of L...
Text Solution
|
- An electron has a spin quantum number +1(1)/(2) and a magnetic quantum...
Text Solution
|
- In photoelectric emission, the energy of the emitted electron is
Text Solution
|
- An electron of mass m and charge -e moves in circular orbit of radius ...
Text Solution
|
- The wave character of moving electron was experimentally verified by :
Text Solution
|
- Which of the following is/are false regarding cathode rays?
Text Solution
|