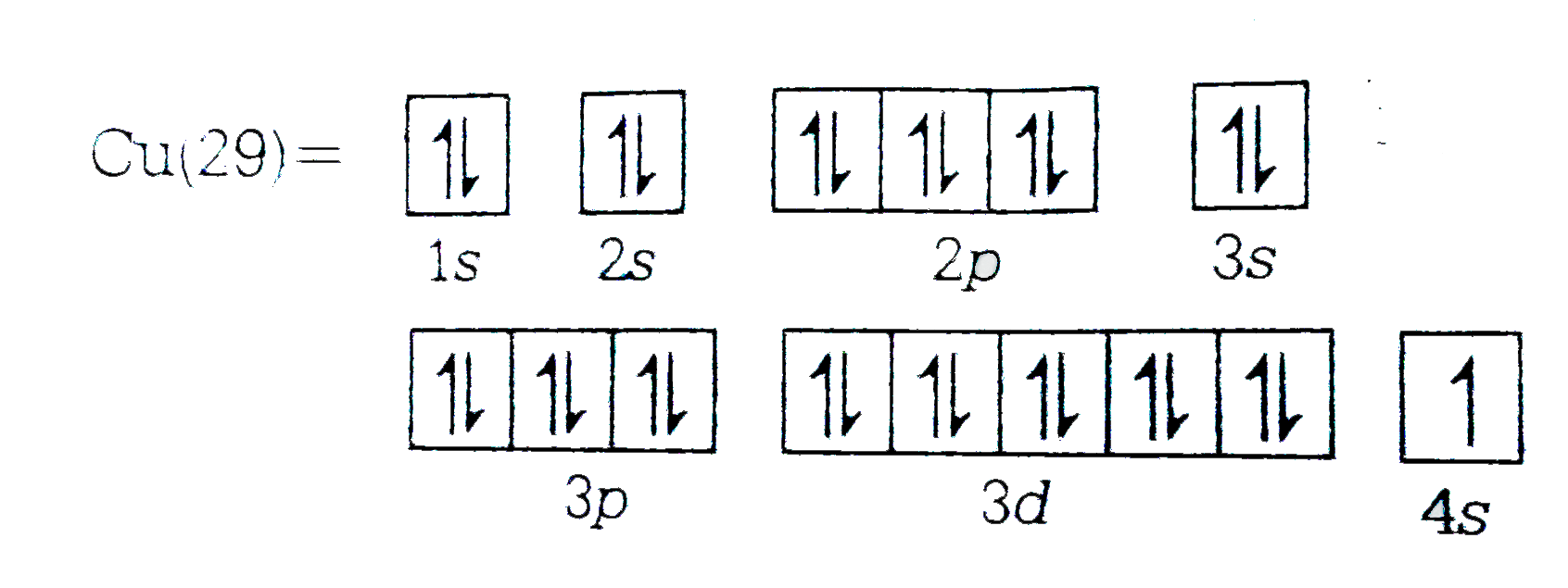A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-ATOMIC STRUCTURE-MCQ
- In photoelectric emission, the energy of the emitted electron is
Text Solution
|
- An electron of mass m and charge -e moves in circular orbit of radius ...
Text Solution
|
- The wave character of moving electron was experimentally verified by :
Text Solution
|
- Which of the following is/are false regarding cathode rays?
Text Solution
|
- For which of the following sets of quantum numbers, an electrons will ...
Text Solution
|
- The ionization potential for hydrogen atom is 13.6 eV, the ionization ...
Text Solution
|
- The energy of an electron in the nth Bohr orbit of hydrogen atom is
Text Solution
|
- Electronic configuration calcium atom can be written as
Text Solution
|
- Electronic configuration of ""(29)Cu in ground state is :
Text Solution
|
- For azimuthal quantum number l=3, the maximum number of electrons wi...
Text Solution
|
- FILLING OF ATOMIC ORBITALS- AUFBAU PRINCIPLE
Text Solution
|
- No two electrons in an atom will have all the four quantum numbers sam...
Text Solution
|
- An ion has 18 electrons in the outermost shell it is
Text Solution
|
- The total number of electrons that can be accommodated in all the orb...
Text Solution
|
- The maximum number of electrons in a subshell is given by the expressi...
Text Solution
|
- Number of unpaired electrons in N^(2+) is/are
Text Solution
|
- Which of the following statement does not form part of Bohr's model o...
Text Solution
|
- If r is radius of first orbit , the radius of nth orbit of the H atom ...
Text Solution
|
- The number of spherical nodes in 3p orbital are
Text Solution
|
- The spectrum of He is expected to be similar to that of
Text Solution
|