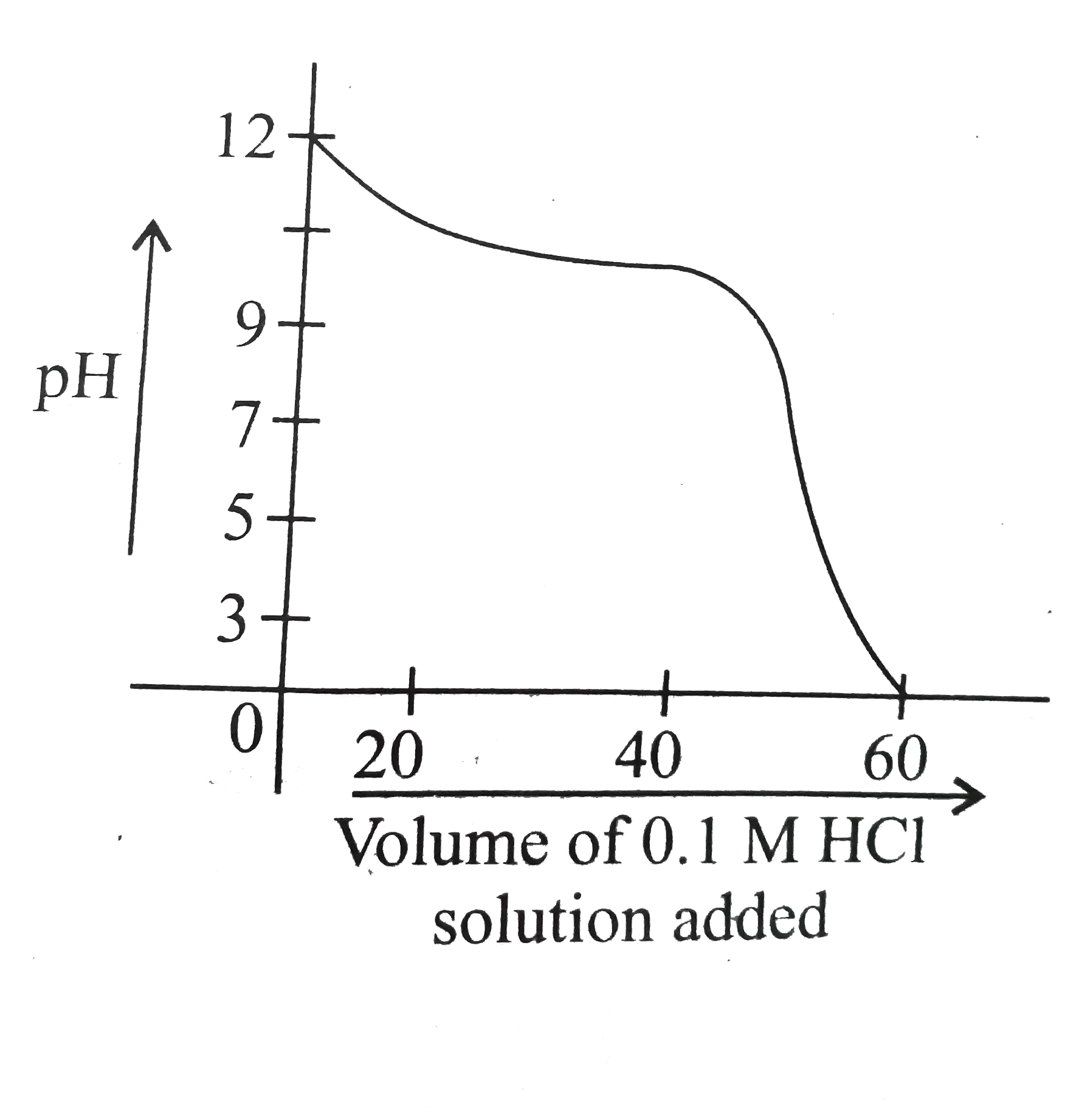
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-IONIC EQUILIBRIUM-partIII one or more than one options correct type
- A buffer solution can be prepared from a mixture of
Text Solution
|
- Aniline behaves as a weak base. When 0.1M ,50mL solution sample of ani...
Text Solution
|
- Which of the following mixtures will act as buffer?
Text Solution
|
- Choose the correct statement(s) about buffer capacity during titration...
Text Solution
|
- Let the colour of the indicator Hin (coloueless) will be visible only ...
Text Solution
|
- When weak base solution (50mL of 0.1 N NH(4)OH) is titrated with stron...
Text Solution
|
- A solution of a substances is titrated against a strong base ( or aci...
Text Solution
|