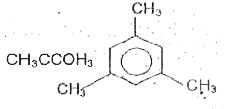
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL - II|24 VideosALDEHYDES AND KETONES
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) COMPREHENSION-I|5 VideosALDEHYDES AND KETONES
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE) LEVEL - II|1 VideosALCOHOLS, ETHERS AND PHENOLS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|7 VideosALKYL AND ARYL HALIDES
FIITJEE|Exercise NUMERICAL BASED QUESTIONS|1 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-ALDEHYDES AND KETONES -ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL - I
- The abstraction of proton will be fastest, in which carbon in the foll...
Text Solution
|
- Which of the following will be most acidic ?
Text Solution
|
- Which of the following will give more enol contents ?
Text Solution
|
- A, B and C are
Text Solution
|
- Product D is,
Text Solution
|
Text Solution
|
- Which of the following will be most readily dehydrated in acidic condi...
Text Solution
|
- Which of the following reduces Tollen's reagent?
Text Solution
|
- CH(3)CN overset(CH(3)MgBr(2" mol"))underset(H(3)O^(+))to A overset("co...
Text Solution
|
- The best method to prepare cyclohexene from cyclohexanol is by using
Text Solution
|
- In Cannizaro reaction which species is best hydride donor (in conc. Al...
Text Solution
|
- The number of pi bond in the product are
Text Solution
|
- The product is
Text Solution
|
- Aliphatic and aromatic aldehydes can be differentiated by the use of
Text Solution
|
- In the reaction sequence A+B overset("NaOH)underset(Delta)to C(6)H(5...
Text Solution
|
- The reduction: can be best carried out by
Text Solution
|
- A mixture of (CH(3))(3)C-CHO and HCHO on heating with aqueous NaOH sol...
Text Solution
|
Text Solution
|
- In the reaction sequence product will be
Text Solution
|
- The most reactive compound towards cyanohydrin Formation is
Text Solution
|