Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE) LEVEL - I (SHORT ANSWER TYPE QUESTIONS)|1 VideosALDEHYDES AND KETONES
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE) LEVEL - I (FILL IN THE BLANKS)|5 VideosALDEHYDES AND KETONES
FIITJEE|Exercise MATCHING TYPE SINGLE CORRECT QUESTION|6 VideosALCOHOLS, ETHERS AND PHENOLS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|7 VideosALKYL AND ARYL HALIDES
FIITJEE|Exercise NUMERICAL BASED QUESTIONS|1 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-ALDEHYDES AND KETONES -SINGLE INTEGER ANSWER TYPE QUESTIONS
- 5-Hydroxyhexanal forms a six member hemiacetal, which predominates at ...
Text Solution
|
- Which hydrogen of the given compound is least acidic in nature ?
Text Solution
|
- Number of carbonyl compounds which form stable geminal diol in below i...
Text Solution
|
- Find the member of monobromo derivatives in the given reaction.
Text Solution
|
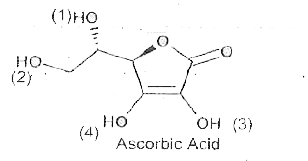
- Ascorbic acid (Vitamin C) does not have a carboxylic group (-COOH) bu...
Text Solution
|
- ""^(14)C of A appears in (B) at…………………..
Text Solution
|
- How many moles of mild oxidizing agent are needed to oxidize the produ...
Text Solution
|
- All possible carbonyl compounds with molecular formula (C(8)H(8)O) whi...
Text Solution
|
- Succinaldehyde on reaction with P(2)O(5) produces organic compound x, ...
Text Solution
|
- The following table shows some laboratory test and their number. ...
Text Solution
|
- Number of haloform form reaction is given by following compounds are x...
Text Solution
|