Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ALKENE, ALKANE & ALKYNE -EXERCISE-5[B]
- Cyclobutyl bromide on treatment with magnesium in dry ether forms an o...
Text Solution
|
- How would you synthesise 4-methoxyphenol from bromobenzene in not mo...
Text Solution
|
- Identify Z + Y in the following synthetic scheme and write their struc...
Text Solution
|
- Compound (X) on reduction with LiAlH(4) gives a hydride (Y) containing...
Text Solution
|
- Mention two esters produced when a recemic mixture of 2-phenyl propano...
Text Solution
|
- Write steps to carry out the conversion of phenol to aspirin.
Text Solution
|
- Convert in not more than four steps. Also mention the reaction con...
Text Solution
|
- Identify (X) and (Y).
Text Solution
|
- When phenyl magnesium bromide reacts with t-bytyl alcohol, the product...
Text Solution
|
- Statement-I: p-Hydroxybenzoic acid has a lower boiling point that o-hy...
Text Solution
|
- In the reaction OCH(3)overset(HBr)rarr the product are:
Text Solution
|
- In the following reacation. The product(s) formed is/are
Text Solution
|
- The acidic hydrolysis of ether (X) shown below is fastest when
Text Solution
|
- The number of hydroxyl group(s) in Q is
Text Solution
|
- Reagent (s) which can be used to bring about the following transformat...
Text Solution
|
- The formation of cyanohydrin from ketone is an example of :
Text Solution
|
- The enolic form of acetone contains
Text Solution
|
- m-Chlorobenzaldehyde on reaction with conc. KOH at room temperature gi...
Text Solution
|
- Hydrogenation of benzoyl chloride in the presence of Pd on BaSO4 gives
Text Solution
|
- An organic compound C(3)H(6)O does not give a precipitate with 2,4-Din...
Text Solution
|
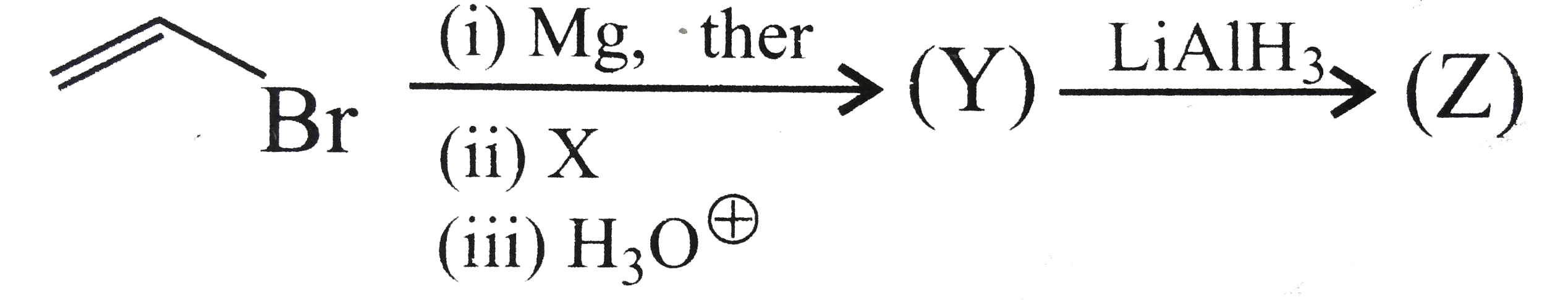 .
.