Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS AND ITS DERIVATIVES
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE )|25 VideosCARBOXYLIC ACIDS AND ITS DERIVATIVES
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE ) (COMPREHENSION)|3 VideosCARBOXYLIC ACIDS AND ITS DERIVATIVES
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|1 VideosCARBOHYDRATES, AMINO ACIDS, POLYMERS AND PRACTICAL ORGANIC CHEMISTRY
FIITJEE|Exercise EXERCISE|4 VideosCHEMICAL ENERGETICS
FIITJEE|Exercise NUMERICAL BASED QUESTIONS|2 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-CARBOXYLIC ACIDS AND ITS DERIVATIVES-SOLVED PROBLEMS (SUBJECTIVE )
- How will you synthesise ? (i) Acetamide from acetone. (ii) lodof...
Text Solution
|
- Complete the following reactions :
Text Solution
|
- The neutralization equivalent of an acid is 116. Fusion of sodim ...
Text Solution
|
- Compound A is acidic in reaction , having the molecular formula , C(4)...
Text Solution
|
- Will the following equations proceed easily ? Explain. (a) CH(3)COC...
Text Solution
|
- How would you bring about the following conversions ? (i) Propi...
Text Solution
|
- An ester C(4)H(8)O(2) (A) on treatment with excess of methyl magnei...
Text Solution
|
- Two moles of an ester (A) are condensed in presence of sodium etho...
Text Solution
|
- An organic acid (A) (C(5)H(10)O(2)) reacts with Br(2) in presence o...
Text Solution
|
- An organic compound C(8)H(10) (A) on treatment with alkaline KMnO(4) ...
Text Solution
|
- The perentage of C, H, N in a disubtituted aromatic compound (A) ...
Text Solution
|
- An alkene (A) on ozonolysis yields acetone and an aldehyde . The al...
Text Solution
|
- Five isomeric para - disubtituted aromatic compounds (A) to (E) wit...
Text Solution
|
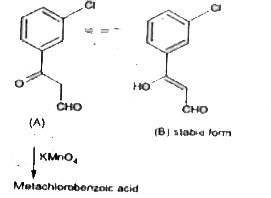
- Compound A of molecular formula C(9)H(7)O(2)Cl exists is keto form and...
Text Solution
|
- A compound (A) has the molecular formula C(6)H(10)O(4). It is a swe...
Text Solution
|