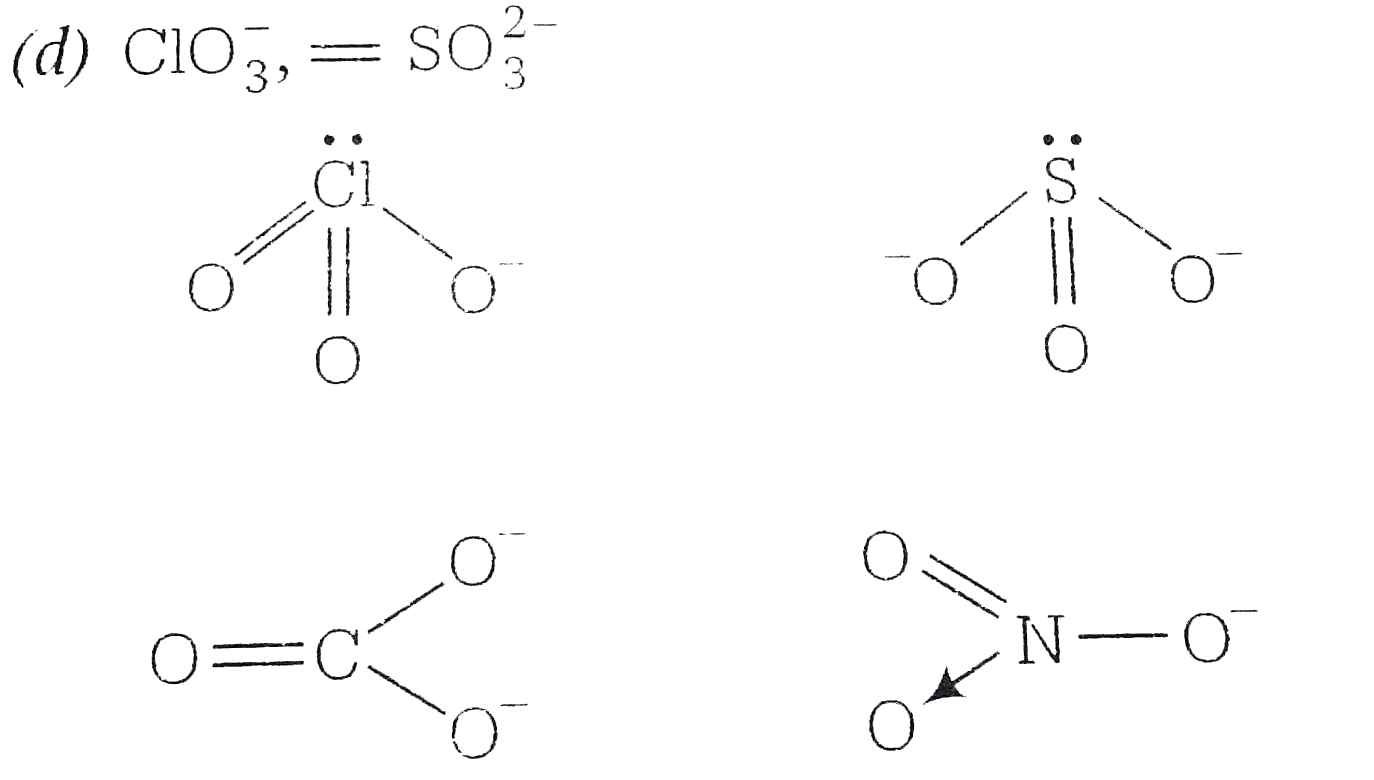
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-CHEMICAL BONDING-EXERCISE
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following species contains equal number of pi and pi bond...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Which of the following options represents the correct bond order ?
Text Solution
|
- Be^(2+) is isoelectronic with which of the following ions ?
Text Solution
|
- Which of the following molecules has the maximum dipole moment ?
Text Solution
|
- Which of the following species has plane tringular shape ?
Text Solution
|
- Indentify the correct order of solubility in aqueous medium
Text Solution
|
- Which one of the following molecules contains no pi - bond ?
Text Solution
|
- Which of the following is a polar molecule
Text Solution
|
- Which of the following is paramagnetic?
Text Solution
|
- Bond order of 1.5 is shown by:
Text Solution
|
- Which of the following species contains three bond pairs and one lone ...
Text Solution
|
- The pair of species with the same bond order is :
Text Solution
|
- Considering the state of hybridization of carbon atoms, find out the m...
Text Solution
|
- Which of the two lons from the list given have the geometry that is ex...
Text Solution
|
- Which of the following is least likely to behave as Lewis acid?
Text Solution
|
- Which of the following has the minimum bond length ?
Text Solution
|
- In which of the following pairs of molecules/ ions, the central atoms ...
Text Solution
|
- Which of the following species does not exist under normal condition...
Text Solution
|