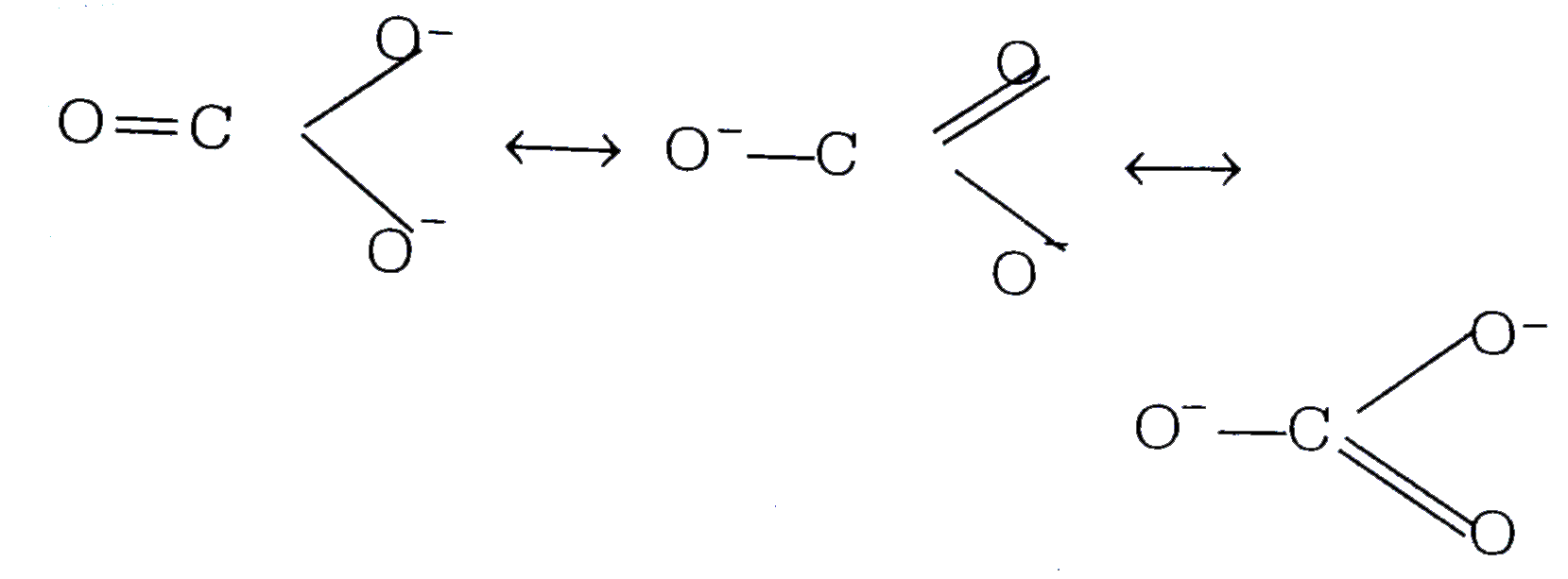
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-CHEMICAL BONDING-EXERCISE
- Angular shape of ozone molecule consists of
Text Solution
|
- In which of the following pairs, the two species are isostructural :
Text Solution
|
- The correct order of increasing C-O bond lengths in CO, CO3^(2-) and ...
Text Solution
|
- Which of the following is not a correct statement ?
Text Solution
|
- The electronegaivity difference between N and F is greater than that b...
Text Solution
|
- Which of the following is not isostructural with SiCI(4) ?
Text Solution
|
- The number of unpaired electrons in a parmamagnetic diatomic molecule ...
Text Solution
|
- Which of the following species has a linear shape?
Text Solution
|
- In which of the following molecules are all the bonds not equal ?
Text Solution
|
- The correct sequence of increasing covalent character is represent by
Text Solution
|
- Which of the following would have permanent dipple moment ?
Text Solution
|
- Which molecule has trigonal planar geometry ?
Text Solution
|
- In BrF(3) molecule, the lone pair occupies equatorial position minimiz...
Text Solution
|
- H2O is dipolar, whereas BeF2 is not. It is because
Text Solution
|
- In an octahedral structure , the pair of d orbitals involved in d^(2)...
Text Solution
|
- In a regular octahedral molecule MX(6) the number of X - M - X bonds ...
Text Solution
|
- Among the following the pair in which the two species are not isostruc...
Text Solution
|
- Which of the following statement is not correct for sigma and pi- bond...
Text Solution
|
- In NO3^- ion, the number of bond pair and lone pair of electrons no N-...
Text Solution
|
- Which of the following has ppi-dpi bonding?
Text Solution
|