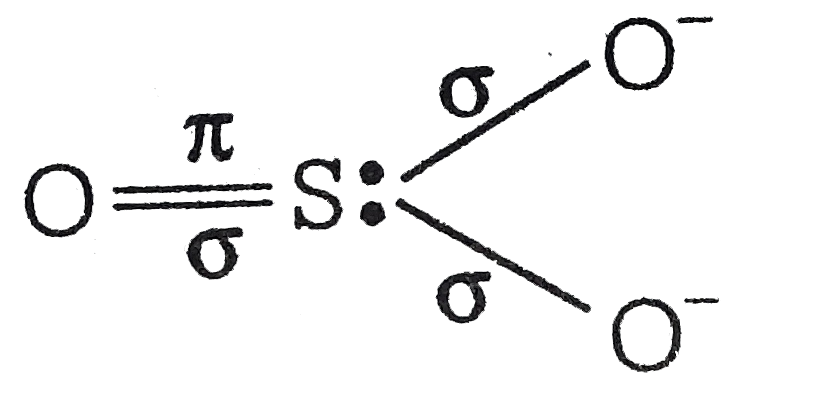
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-CHEMICAL BONDING-EXERCISE
- Which of the following statement is not correct for sigma and pi- bond...
Text Solution
|
- In NO3^- ion, the number of bond pair and lone pair of electrons no N-...
Text Solution
|
- Which of the following has ppi-dpi bonding?
Text Solution
|
- Which of the following is isoelectronic ?
Text Solution
|
- The main axis of diatomic molecule is z. The orbitals px and py overla...
Text Solution
|
- In X - H----Y , both X and Y are electronegative elements
Text Solution
|
- In which of the following bond angle is maximum
Text Solution
|
- Which of the following two are isostructural ?
Text Solution
|
- A compound contains three elements A,B and C, if the oxidation number ...
Text Solution
|
- Among the following ions the p pi- d pi overlap could be present in
Text Solution
|
- Among the following group which represents the collection of isoelectr...
Text Solution
|
- Which of the following is not paramagnetic ?
Text Solution
|
- The relationship between the dissociation energy of N2 and N2^+ is
Text Solution
|
- Which one of the following is planar ?
Text Solution
|
- Which of the following molecule forms linear polymeric structure due t...
Text Solution
|
- The type of hybridisation of boron in diborane is
Text Solution
|
- In PO(4)^(3-) the formal charge on each O-atom and P-O bond order resp...
Text Solution
|
- The species which is not paramagnetic among the following is
Text Solution
|
- The number of antibonding electron pairs in O(2)^(2-) molecular ion on...
Text Solution
|
- The molecule which does not exhibit dipole moment is
Text Solution
|