A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-CHEMICAL BONDING-EXERCISE
- BCl3 molecule is planar while NCl3 is pyramidal because
Text Solution
|
- Which of the following species is paramagnetic ?
Text Solution
|
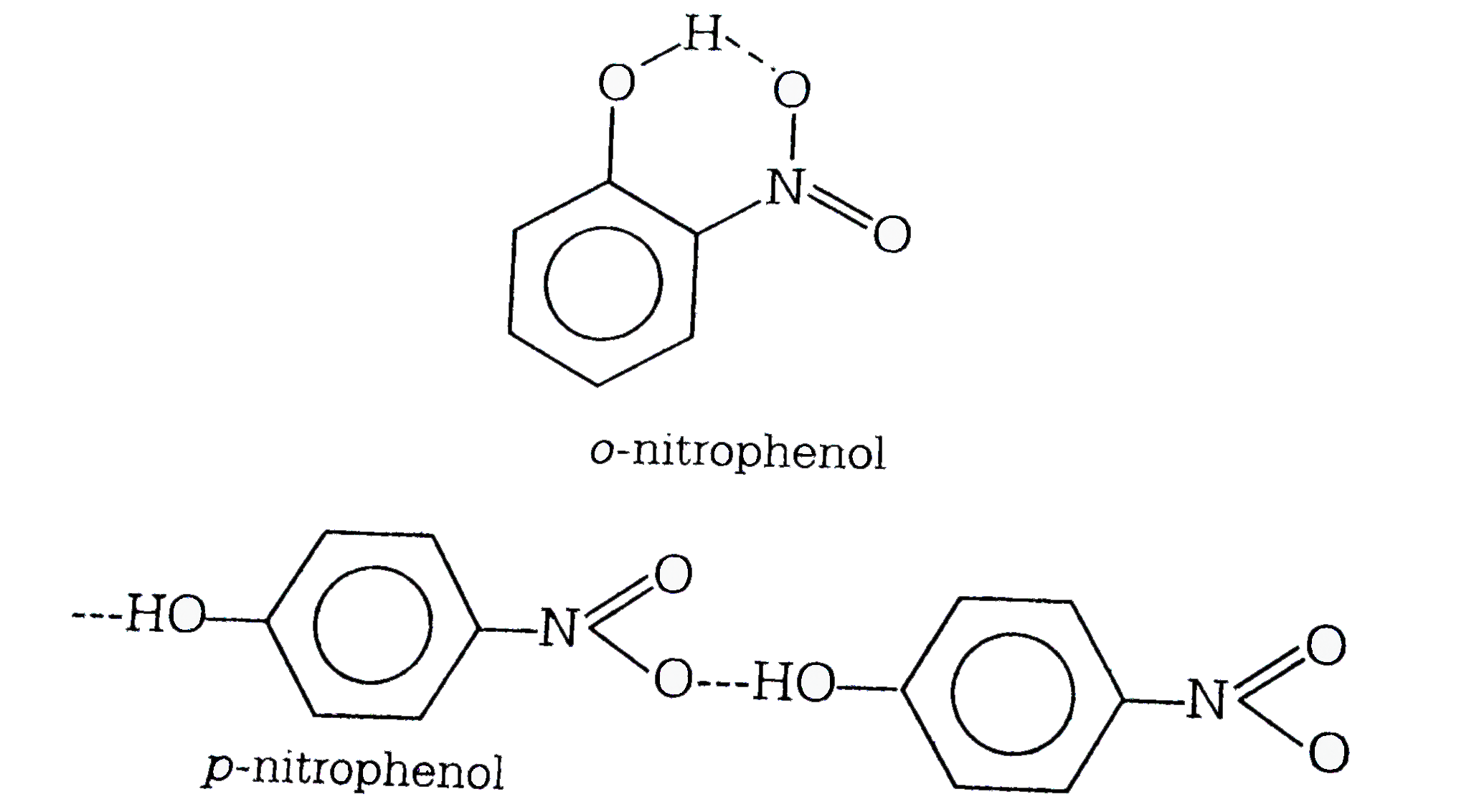
- The boiling point of p-nitrophenol is higher than that of o-nitropheno...
Text Solution
|
- Linus Pauling received the Nobel Prize for his work on
Text Solution
|
- Among the following orbital bonds, the angle is minimum between
Text Solution
|
- Which of the following pairs will form the most stable ionic bond ?
Text Solution
|
- Which of the following does not have a tetrahedral structure ?
Text Solution
|
- Which is the weakest among the following types of bonds
Text Solution
|
- Mark the incorrect statement in the following .
Text Solution
|
- The dielectric constant of H(2)O is 80. The electrostatic force of att...
Text Solution
|
- When the hybridization state of a carbon atom changes from sp^(3) to s...
Text Solution
|
- Which of the following statement is not correct ?
Text Solution
|
- Which one of the following is the correct order of interactions ?
Text Solution
|
- Which compound will show the highest lattice energy ?
Text Solution
|
- strongest hydrogen bonding is shown by
Text Solution
|
- Which structure is linear ?
Text Solution
|
- An sp^(3) hybrid orbital possesses
Text Solution
|
- Which one of the following formulae does not correctly represent the b...
Text Solution
|
- Linear combination of two hybridised orbitals belonging to the two ato...
Text Solution
|
- Which one shows maximum hydrogen bonding ?
Text Solution
|