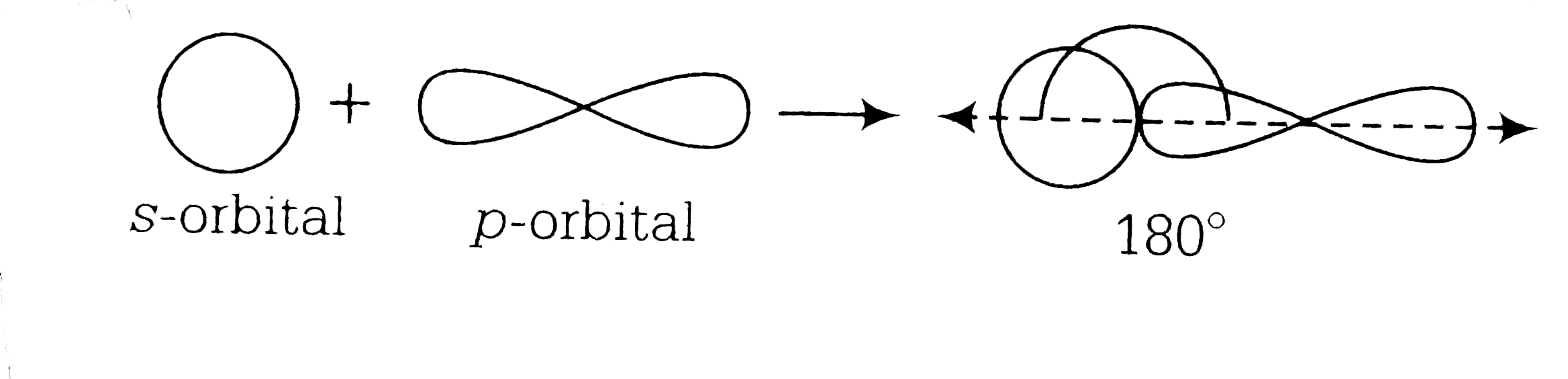
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-CHEMICAL BONDING-EXERCISE
- Which is the weakest among the following types of bonds
Text Solution
|
- Mark the incorrect statement in the following .
Text Solution
|
- The dielectric constant of H(2)O is 80. The electrostatic force of att...
Text Solution
|
- When the hybridization state of a carbon atom changes from sp^(3) to s...
Text Solution
|
- Which of the following statement is not correct ?
Text Solution
|
- Which one of the following is the correct order of interactions ?
Text Solution
|
- Which compound will show the highest lattice energy ?
Text Solution
|
- strongest hydrogen bonding is shown by
Text Solution
|
- Which structure is linear ?
Text Solution
|
- An sp^(3) hybrid orbital possesses
Text Solution
|
- Which one of the following formulae does not correctly represent the b...
Text Solution
|
- Linear combination of two hybridised orbitals belonging to the two ato...
Text Solution
|
- Which one shows maximum hydrogen bonding ?
Text Solution
|
- Among LiCI,BeCI(2) and C CI(4) the covalent bond character varies as .
Text Solution
|
- H2O has net dipole moment while BeF2 has zero dipole moment because
Text Solution
|
- In which one of the following molecules , the central atom said to ado...
Text Solution
|
- Which of the following does not apply to metallic bond ?
Text Solution
|
- Which of the following molecule does not have a linear arrangement of ...
Text Solution
|
- The equilateral shape has
Text Solution
|
- The angle between the overlapping of one s-orbital and one p-orbital i...
Text Solution
|