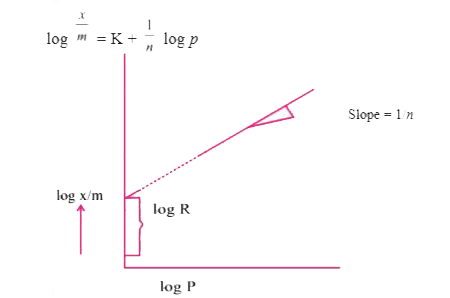
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-PRACTICE - PAPER-Section : C
- 2 g of benzoic acid (C(6) H(5) COOH) dissolved in 25 g of benzene show...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- Explain what is observed? (i) When a beam of light is passed through...
Text Solution
|
- Draw the figure to show the splitting of d-orbitals in an octahedral c...
Text Solution
|
- Carry out the following conversions: (i) Aniline to chlorobenzene ...
Text Solution
|
- Write short on the following : (i) Carbylamine reaction (ii) Hofma...
Text Solution
|