Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS|3 VideosCOORDINATION COMPOUNDS
CBSE COMPLEMENTARY MATERIAL|Exercise SHORT ANSWER-I TYPE QUESTIONS|18 VideosCHEMISTRY IN EVERYDAY LIFE
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS|2 VideosELECTROCHEMISTRY
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS (5 Marks)|7 Videos
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-COORDINATION COMPOUNDS-SHORT ANSWER-II TYPE QUESTIONS
- A coordiantion compound has the formula CoCl(3).4NH(3). It does not li...
Text Solution
|
- Why does a tetrahedral complex of the type [MA(2)B(2)] not show geomet...
Text Solution
|
- The molar conductivity of the complex CoCl(3).4NH(3).2H(2)O is found t...
Text Solution
|
- [Ti(H(2)O)(6)]^(3+) is coloured while [Sc(H(2)O)(6)]^(3+) is colourl...
Text Solution
|
- Describe with an example of each, the role of coordination compounds i...
Text Solution
|
- Write the type of isomerism exhibited by the following complexes: (i)...
Text Solution
|
- Explain the following: (i) CO is stronger ligand than NH(3). (ii)...
Text Solution
|
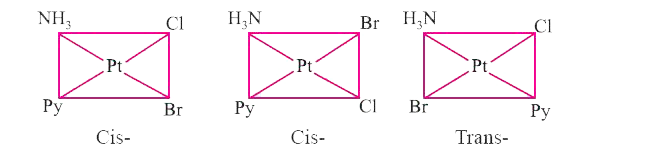
- Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how man...
Text Solution
|