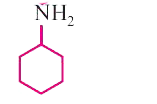
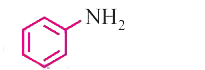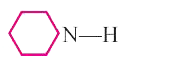A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
CBSE COMPLEMENTARY MATERIAL|Exercise MCQ (Assertion and Reason)|2 VideosAMINES
CBSE COMPLEMENTARY MATERIAL|Exercise MCQ (Matching)|2 VideosALDEHYDES,KETONES AND CARBOXYLIC ACIDS
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS (5 MARKS)|21 VideosBIOMOLECULES
CBSE COMPLEMENTARY MATERIAL|Exercise SHORT ANSWER-II TYPE QUESTION|13 Videos
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-AMINES-5 MARKS
- Which is the weakest base :
Text Solution
|
- Arrange the following : (i) In decreasing order of pKb values : C(...
Text Solution
|
- How will you convert : (i) Ethanoic acid into methanamine (ii) Hexa...
Text Solution
|
- Write short note on the following : (i) Carbylamine reaction (ii) D...
Text Solution
|
- Complete the following reactions : (i) C(6)H(5)NH(2) + H(2)SO(4) (co...
Text Solution
|
- Write A,B and C in the given reactions : (i) C(6)H(5)N(2)CI overset(...
Text Solution
|
- Accomplish the following conversions : (i) C(6)H(5)NO(2) rightarrow...
Text Solution
|
- Give reasons for the following (a) Acetylation of aniline reduces it...
Text Solution
|