Text Solution
Verified by Experts
Topper's Solved these Questions
UNSATURATED HYDROCARBONS
OP TANDON|Exercise PROBLEMS FOR PRACTICE|17 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise Match type|1 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|10 VideosSTOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise SELF ASSESSMENT|11 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-UNSATURATED HYDROCARBONS-SOME SOLVED PROBLEMS
- Give the product(s) of reductive ozonolysis of the following compounds...
Text Solution
|
- Write the structure of the isomeric butenes and name them. Give the pr...
Text Solution
|
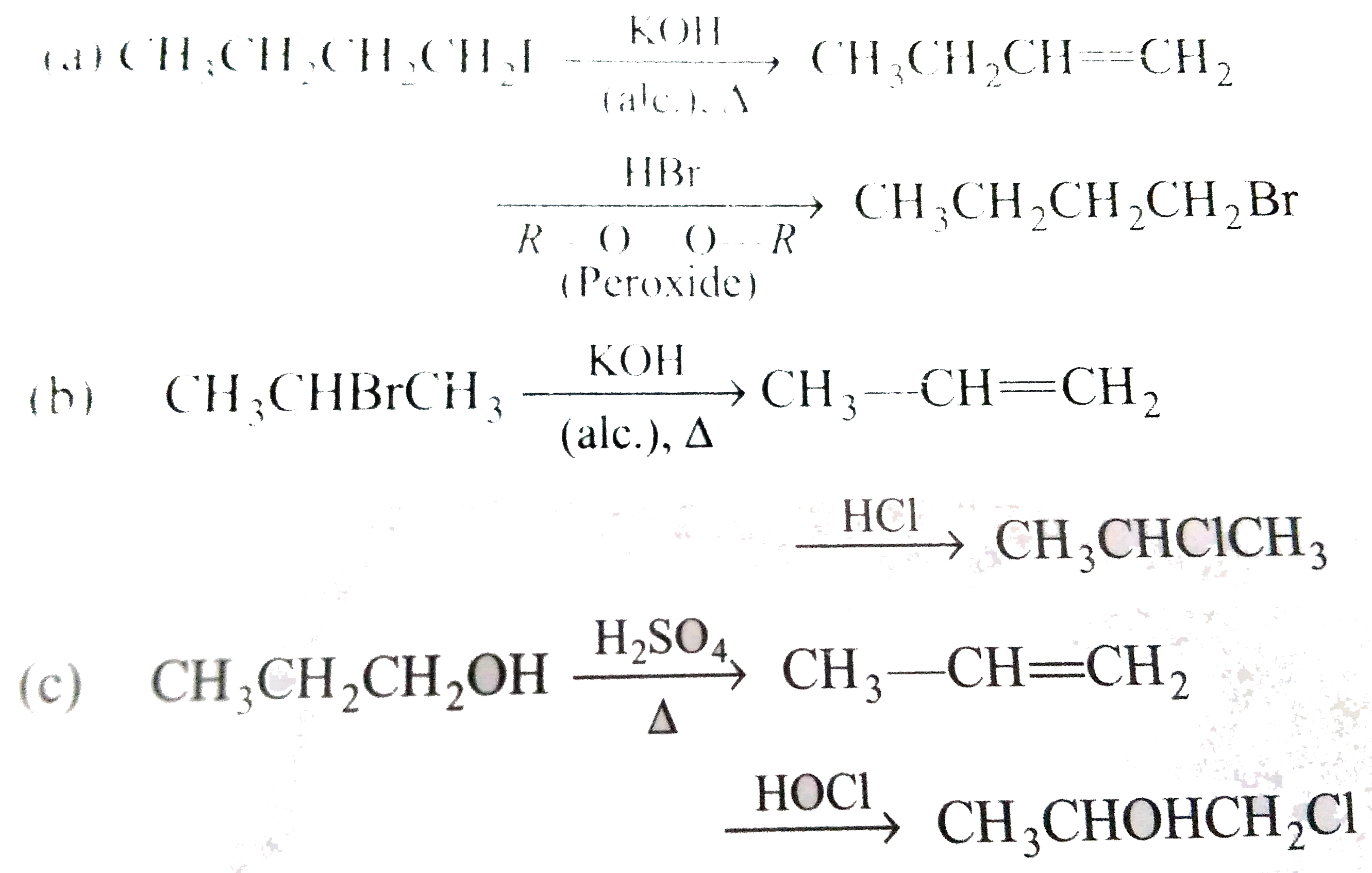
- Effect the following transformations: a) CH(3)CH(2)CH(2)I to CH(3)CH...
Text Solution
|
- Account for the following: a) The C-=C distance is shorter than C=C ...
Text Solution
|
- Write the structural and stereoisomers of pentene, C(5)H(10) and compa...
Text Solution
|
- What are the possible products, in order of decreasing yield, obtained...
Text Solution
|
- Prepare the following from 1-pentene: i) CH(3)CH(2)CH(2)DCH(3) (A) ...
Text Solution
|
- Give the products formed by the oxidation of hot KMnO(4). a. C(4)H(...
Text Solution
|
- Predict the product of the reaction,
Text Solution
|
- How will you synthesis the following? i) Hexa-1,5-diene from propene...
Text Solution
|
- Explain the following: i) Beta-1,3-diene gives 1,2- and 1,4-addition...
Text Solution
|
- i) An organic compound, C(4)H(6), on ozonolysis gives formaldehyde and...
Text Solution
|
- a) Draw the stereochemical structure of the product in the following r...
Text Solution
|
- Indicate allylic, vinyl, 1^(@), 2^(@),3^(@) hydrogen in the following ...
Text Solution
|
- Hydrocarbon (X), C(7)H(12), on reaction with boron hydride followed by...
Text Solution
|
- Give structures/configuration of the products in the following reactio...
Text Solution
|
- A hydrocarbons C(4)H(8), neither decolourised bromine in carbon tetrac...
Text Solution
|
- Give the structures of the major products from 3-ethylpent-2-ene under...
Text Solution
|
- Give the major product (A) in the following reaction:
Text Solution
|
- Identify (A) to (F) :
Text Solution
|