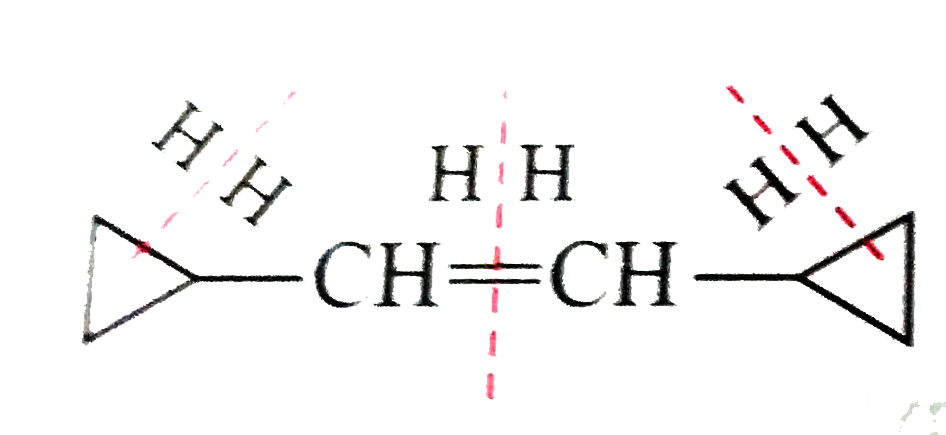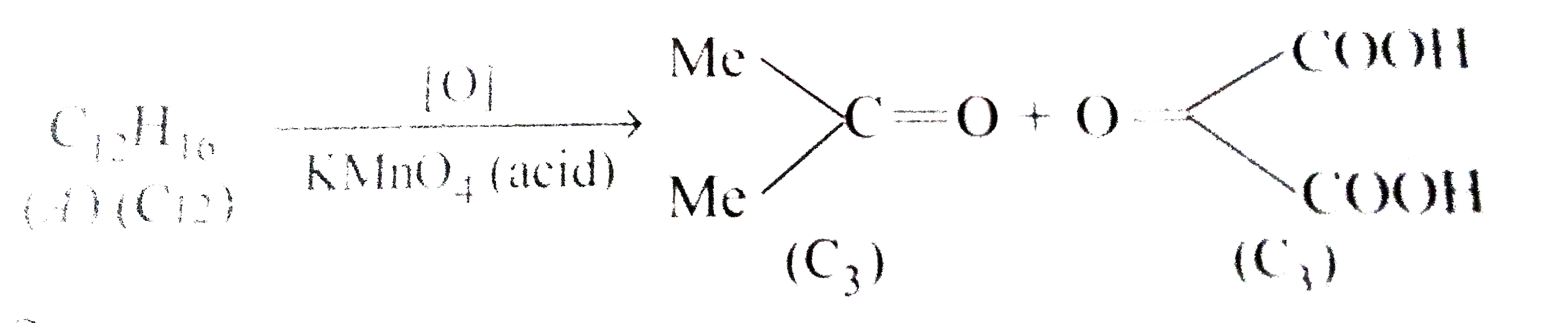Text Solution
Verified by Experts
Topper's Solved these Questions
UNSATURATED HYDROCARBONS
OP TANDON|Exercise Match type|1 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise PROBLEMS BASED ON STRUCTURE AND PROPERTIES|10 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise SOME SOLVED PROBLEMS|31 VideosSTOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise SELF ASSESSMENT|11 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-UNSATURATED HYDROCARBONS-PROBLEMS FOR PRACTICE
- Explain the formation of products (B), (C), and (D) in the following r...
Text Solution
|
- Complete the following equations: a) H(2)C=CH-CH(3)+HCl to (A) b) ...
Text Solution
|
- Draw all the structural , stereo, and cyclic isomers of C(4) H(6).
Text Solution
|
- a) Give the structural formulae of the alkene (C(8)H(16)) which on red...
Text Solution
|
- Answer the following: a) If the bond order (number of bonds) between...
Text Solution
|
- a) Arrange benzene, n-hexane and ethyne in decreasing order of acidic ...
Text Solution
|
- Draw the cis and trans structures of hex-2-ene. Which isomer will have...
Text Solution
|
- Two compounds (X) and (Y) of the same molecular formula. C(4)H(6) gave...
Text Solution
|
- Identify the products of the following reactions:
Text Solution
|
- How will you synthesis? ltbrlt a) Vinyl chloride from acetylene b) B...
Text Solution
|
- How will you obtain the following from propene? a) n-Propyl bromide ...
Text Solution
|
- How will you differentiate the following? A) Ethylene and ethane b) ...
Text Solution
|
- a) A gaseous mixutre consists ethane and ehylene. How are the individu...
Text Solution
|
- Explain the mechanism of the following reactions: a) Addition of bro...
Text Solution
|
- Deduce the structures of the compounds which yeild the following produ...
Text Solution
|
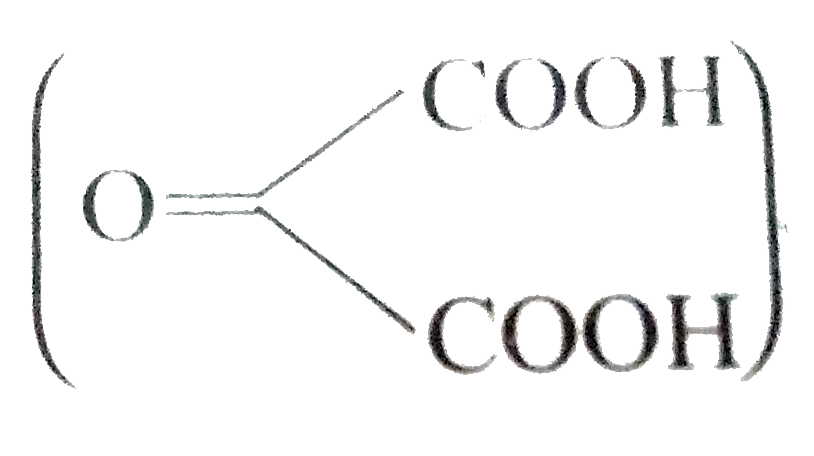
- a) Compound (A) C(8)H(12) on oxidation gives an acid (B) C(4)H(6)O(2)....
Text Solution
|
- Explain the following: a) Why alkenes are more reactive than alkanes...
Text Solution
|