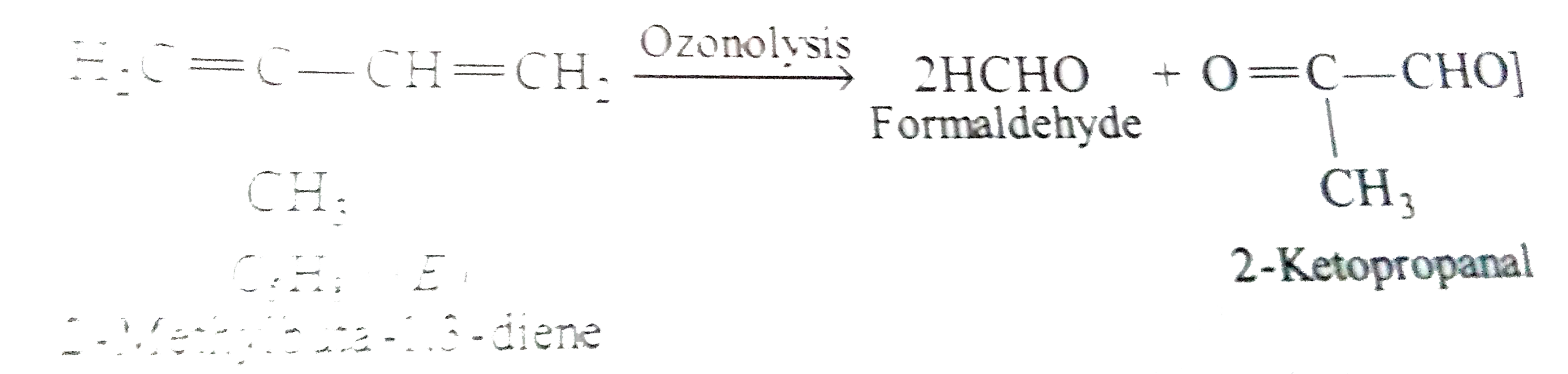Text Solution
Verified by Experts
Topper's Solved these Questions
UNSATURATED HYDROCARBONS
OP TANDON|Exercise BRAIN STORMING PROBLEMS|19 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise OBJECTIVE QUESTIONS|194 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise Match type|1 VideosSTOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise SELF ASSESSMENT|11 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-UNSATURATED HYDROCARBONS-PROBLEMS BASED ON STRUCTURE AND PROPERTIES
- An organic compound [A] C(6)H(10), on reduction first gives [B] C(6)H(...
Text Solution
|
- (A), (B) and ( C) are isomeric heptenes, (A) on ozonolysis gives ethan...
Text Solution
|
- An organic compound (E)(C(5)H(8)), on hydrogenation gives a compound ...
Text Solution
|
- Compound (A) on treatment with NaNH(2) followed by CH(3)CH(2)Br gave c...
Text Solution
|
- A hydrocarbon (A) of the formula C(7)H(12) on ozonolysis gives a compo...
Text Solution
|
- Hydrocarbon (A) C(6)H(10), on treatment with H(2)//Ni, H(2)/ Lindlar's...
Text Solution
|
- Dehydration of (A) with conc. H(2)SO(4) gives a compound that exists i...
Text Solution
|
- What are the possible isomers of formula C(7)H(12) which gives above p...
Text Solution
|
- Identify (X), (Y) and (Z) in the following synthetic scheme and write ...
Text Solution
|
- An alkane (A) C(16)H(16) on ozonolysisi gives only one products (B) C(...
Text Solution
|