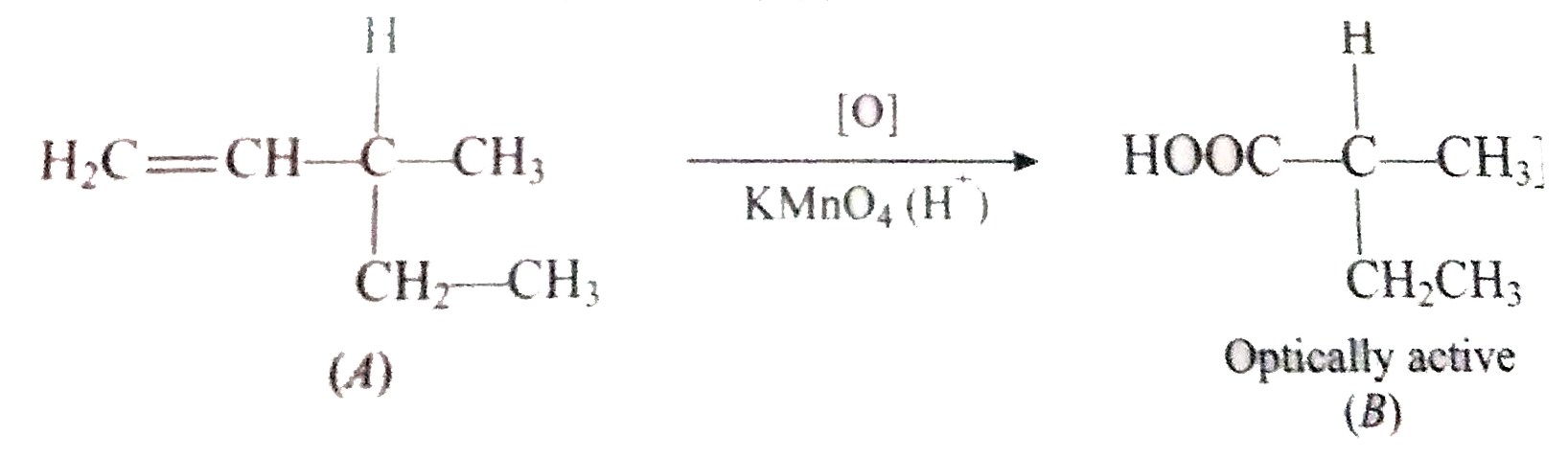Text Solution
Verified by Experts
Topper's Solved these Questions
UNSATURATED HYDROCARBONS
OP TANDON|Exercise OBJECTIVE QUESTIONS|194 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise (LEVEL B) OBJECTIVE QUESTIONS|29 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise PROBLEMS BASED ON STRUCTURE AND PROPERTIES|10 VideosSTOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise SELF ASSESSMENT|11 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-UNSATURATED HYDROCARBONS-BRAIN STORMING PROBLEMS
- Write down the products when following compounds are dehydrated:
Text Solution
|
- Write down dehydration products of the following compound.
Text Solution
|
- Complete the following reactions:
Text Solution
|
- Complete the following reactions:
Text Solution
|
- Give the products with their stereoisomers, if any. a) Me--=-Me over...
Text Solution
|
- Give the structures of components which give following products on hyd...
Text Solution
|
- An unsaturated compound C(6)H(12) A) decolourless Br(2). Water and on ...
Text Solution
|
- A compound C(5)H(9)Br (A) does not decolourie Baeyer's reagent or Br(2...
Text Solution
|
- Give the structure of polyisoprene (natural rubber). Write down the oz...
Text Solution
|
- Limonene (C(10)H(16)) is a naturally occuring hydrocarbon. It absorbs ...
Text Solution
|
- Hydrocarbon (A) (C=87.2%) on hydrogenation forms (B) (C=84.1%). Ozonol...
Text Solution
|
- C(11)H(16) (A) reacts with two equivalent of H(2) and on reductive ozo...
Text Solution
|
- Complete the following reaction:
Text Solution
|
- a) Identify the product in the following reactions: b) Give the p...
Text Solution
|
- a) Identify the products among the following:
Text Solution
|
- Explain why the addition of HI to 3,3-dimethylbut-1-ene gives 2-iodo-2...
Text Solution
|
- Complete the following reactions:
Text Solution
|
- Complete the following reactions:
Text Solution
|
- Complete the following reactions:
Text Solution
|