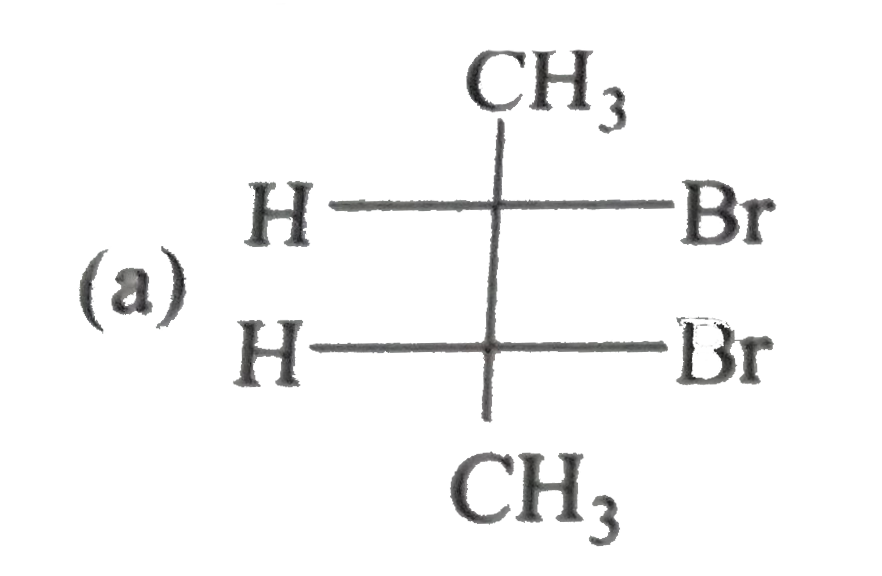
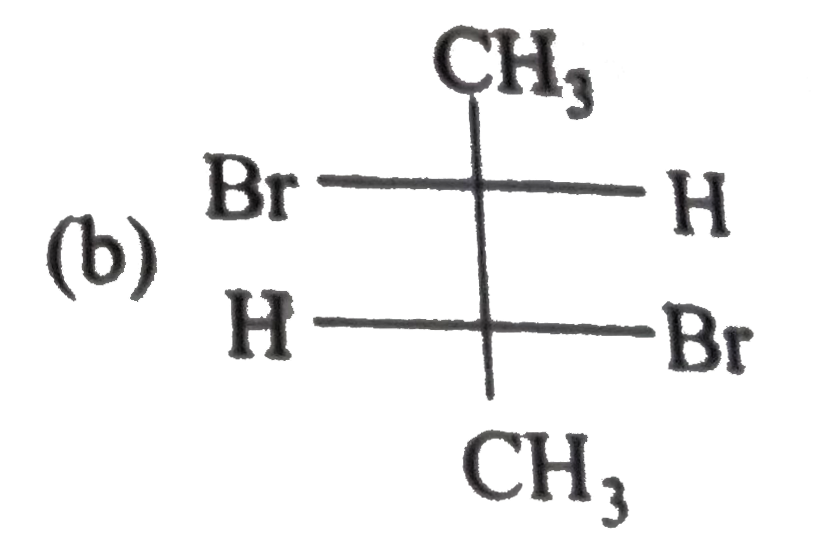
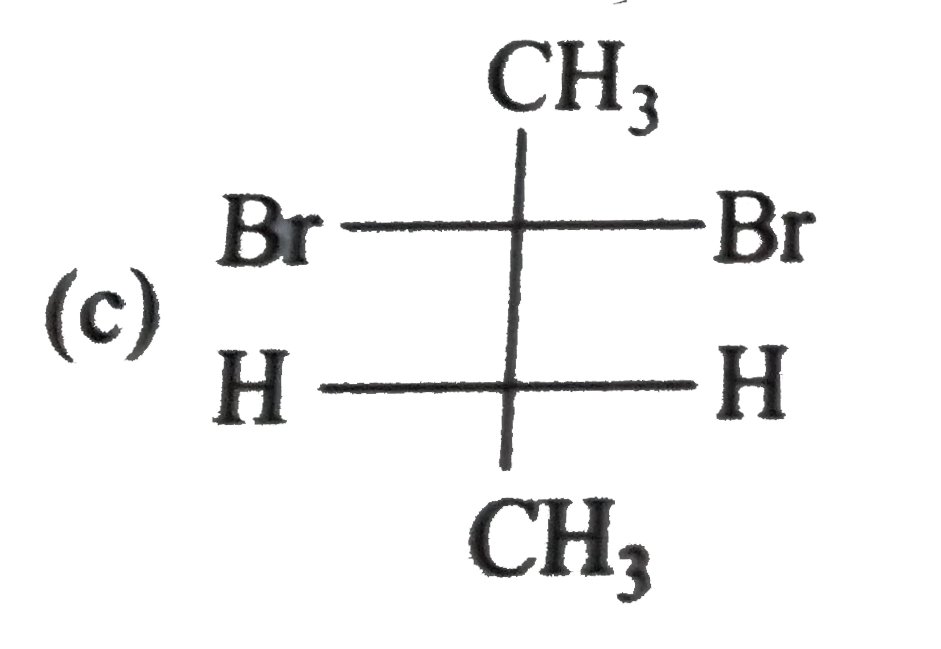
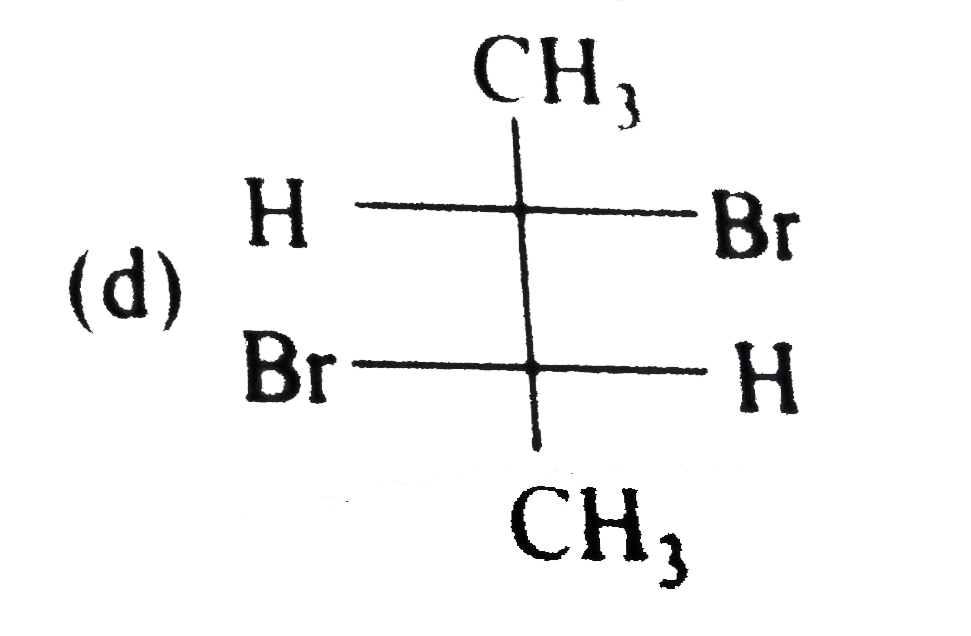
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
UNSATURATED HYDROCARBONS
OP TANDON|Exercise (LEVEL B) OBJECTIVE QUESTIONS|29 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise SET II This set contains the questions with more ..|23 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise BRAIN STORMING PROBLEMS|19 VideosSTOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise SELF ASSESSMENT|11 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-UNSATURATED HYDROCARBONS-OBJECTIVE QUESTIONS
- HBr reacts with H(2)C=CH-OCH(3) under anhydrous conditions at room tem...
Text Solution
|
- Which product is formed when trans-2-phenyl-1-bromocyclopentane is tre...
Text Solution
|
- trans-2-Butene +Br(2) gives:
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with Markowni...
Text Solution
|
- Which of the following reagents, which heated with ethyl chloride, for...
Text Solution
|
- Ozonolysis products of an olefin are OHC-CHO and OHC-CH(2)-CH(2)-CHO, ...
Text Solution
|
- What is formed when calcium carbide reacts with heavy water?
Text Solution
|
- A molecule (X) has (i) four sigma bonds formed by hte overlap of sp^(2...
Text Solution
|
- RCH=CH(2) overset(Na//NH(2)(l))underset(C(2)H(5)OH)to RCH(2)CH(3) Th...
Text Solution
|
- CH(3)CH=CH(2)+NOClrarrP Identify the product.
Text Solution
|
- Identitfy 'B' in the following reactions: H(2)C=CH(2)+HCl overset("A...
Text Solution
|
- The product C is CH(3).CH(2).C-=CH+HCl rarrBoverset(HI)rarrC
Text Solution
|
- CaC(2)+H(2)O to (X) overset(O(3)//H(2)O)toHCOOH, (X) is:
Text Solution
|
- B overset(Lindlar's)underset(Catalyst)larrR-C=C-Roverset(Na//Nh(3))rar...
Text Solution
|
- The reagent(s) for the following conversion, is/are:
Text Solution
|
- Arrange the following according to the increasing order of stability: ...
Text Solution
|
- Identify the products in the following reaction 3CH(3)CH=CH(2)overse...
Text Solution
|
- A hydrocarbon of molecular formula, C(6)H(10) reacts with sodamine and...
Text Solution
|
- 2,3-Dimethyl-2-butene can be prepared by heating which of the followin...
Text Solution
|
- What will be the increasing order of the stability of the following al...
Text Solution
|