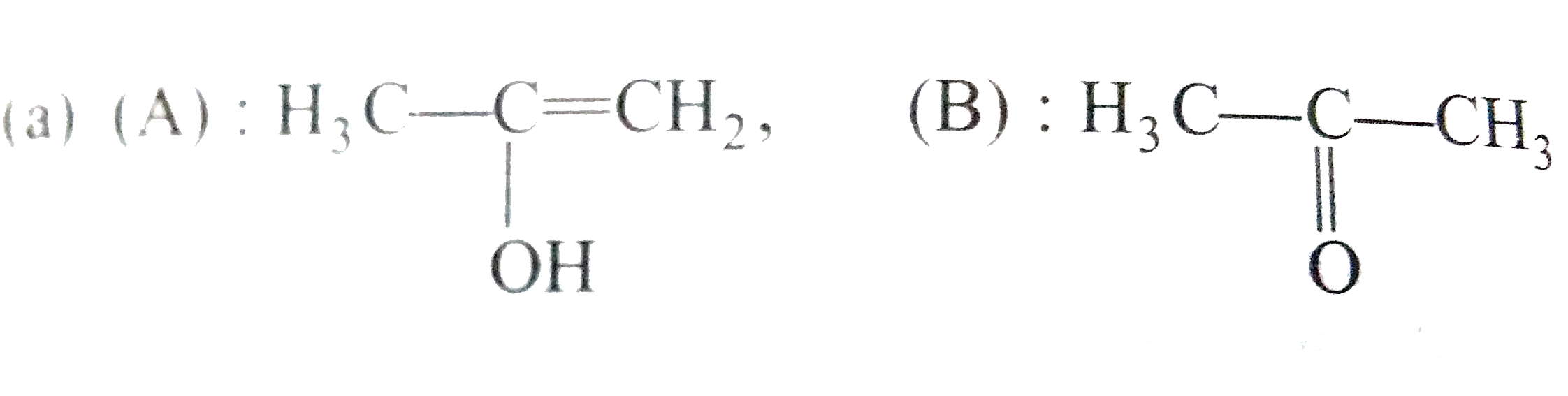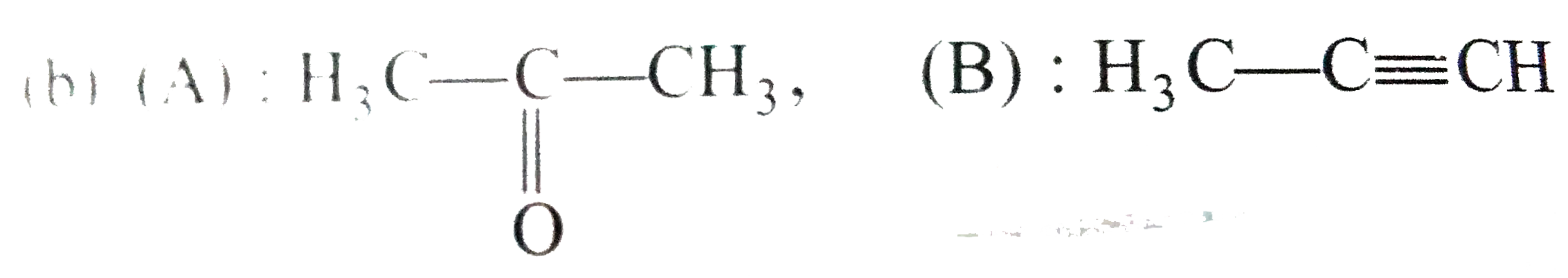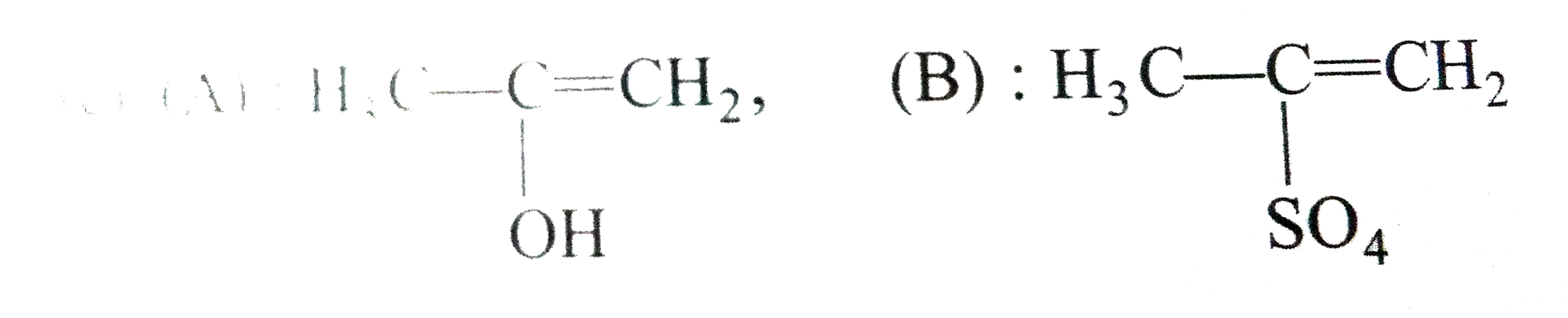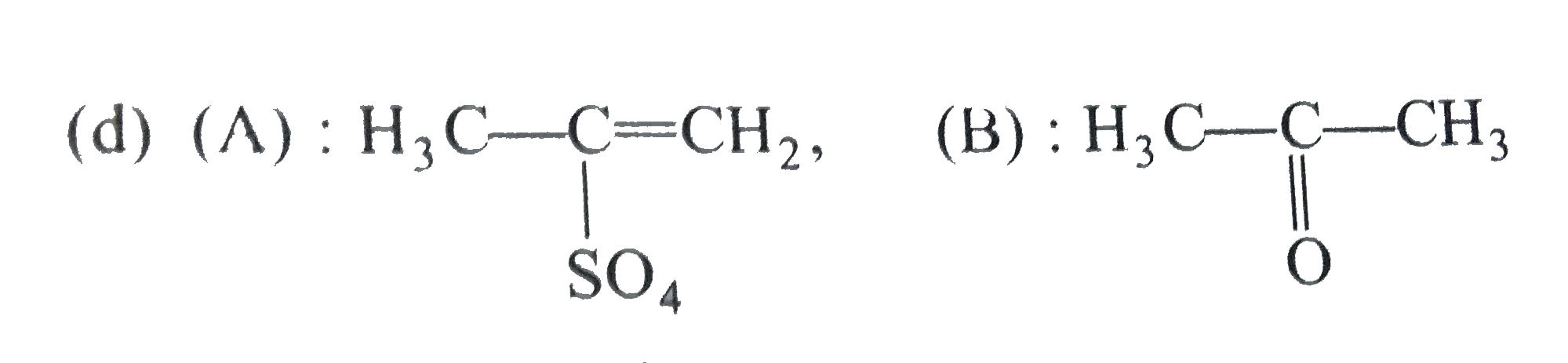A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
UNSATURATED HYDROCARBONS
OP TANDON|Exercise (LEVEL B) OBJECTIVE QUESTIONS|29 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise SET II This set contains the questions with more ..|23 VideosUNSATURATED HYDROCARBONS
OP TANDON|Exercise BRAIN STORMING PROBLEMS|19 VideosSTOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )
OP TANDON|Exercise SELF ASSESSMENT|11 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-UNSATURATED HYDROCARBONS-OBJECTIVE QUESTIONS
- One mole of a symmetrical alkene on ozonolysis gives two moles of an a...
Text Solution
|
- The decreasing order of acidic character among ethane(I), ethene(II), ...
Text Solution
|
- Predict the correct intermediate and product in the following reaction...
Text Solution
|
- The number of optically active products obtained from the complete ozo...
Text Solution
|
- In the following reaction, the product 'R' is: CaC(2) overset(H(2)O)...
Text Solution
|
- Name the reagent used to bring about the following transformation, but...
Text Solution
|
- Identify the product in the reaction Ph-C-=kC-Meoverset(H(3)O^(+), H...
Text Solution
|
- The major product formed when 2-bromo-methylbutane is refluxed with et...
Text Solution
|
- Identify Z in the sequence of reactions : CH(3)CH(2)CH=CH(2)underse...
Text Solution
|
- The synthesis of 3-octyne is achieved by adding a bromoalkane into a m...
Text Solution
|
- Products of the reactions:
Text Solution
|
- The major organic compound formed by the reaction of 1,1,1-trichloroet...
Text Solution
|
- The reagents to carry out of the following conversion are :
Text Solution
|
- When but-2-yne is treated with Na in liquid ammonia:
Text Solution
|
- The products obtained by the ozonolysis of 2-ethyl but-1-ene are:
Text Solution
|
- In the following sequential transformation, considering only the major...
Text Solution
|
- In the following sequential transformation, considering only the major...
Text Solution
|
- The compound which would yield 5- Oxo-2-methylhexanal on reductive ozo...
Text Solution
|
- In the following reaction , the major product is :
Text Solution
|
- The reaction of propene with HOCI (CI(2)+H(2)O) proceeds through the i...
Text Solution
|