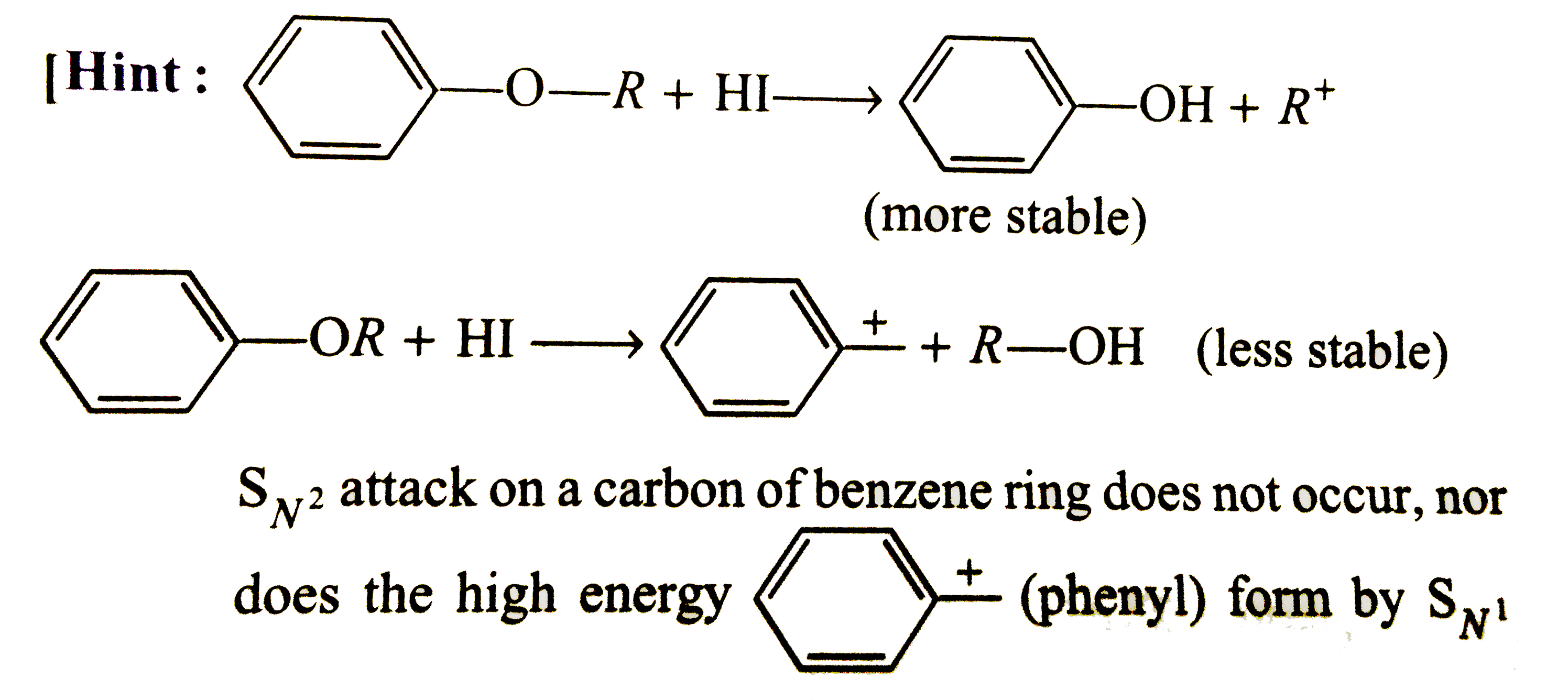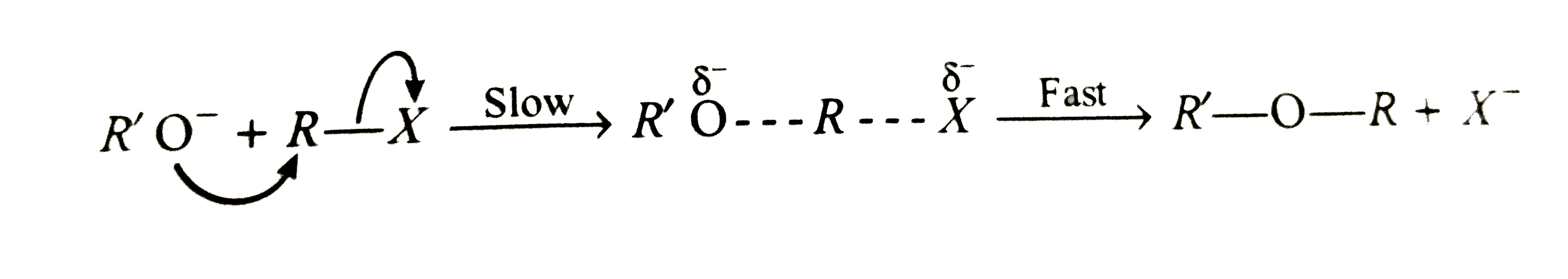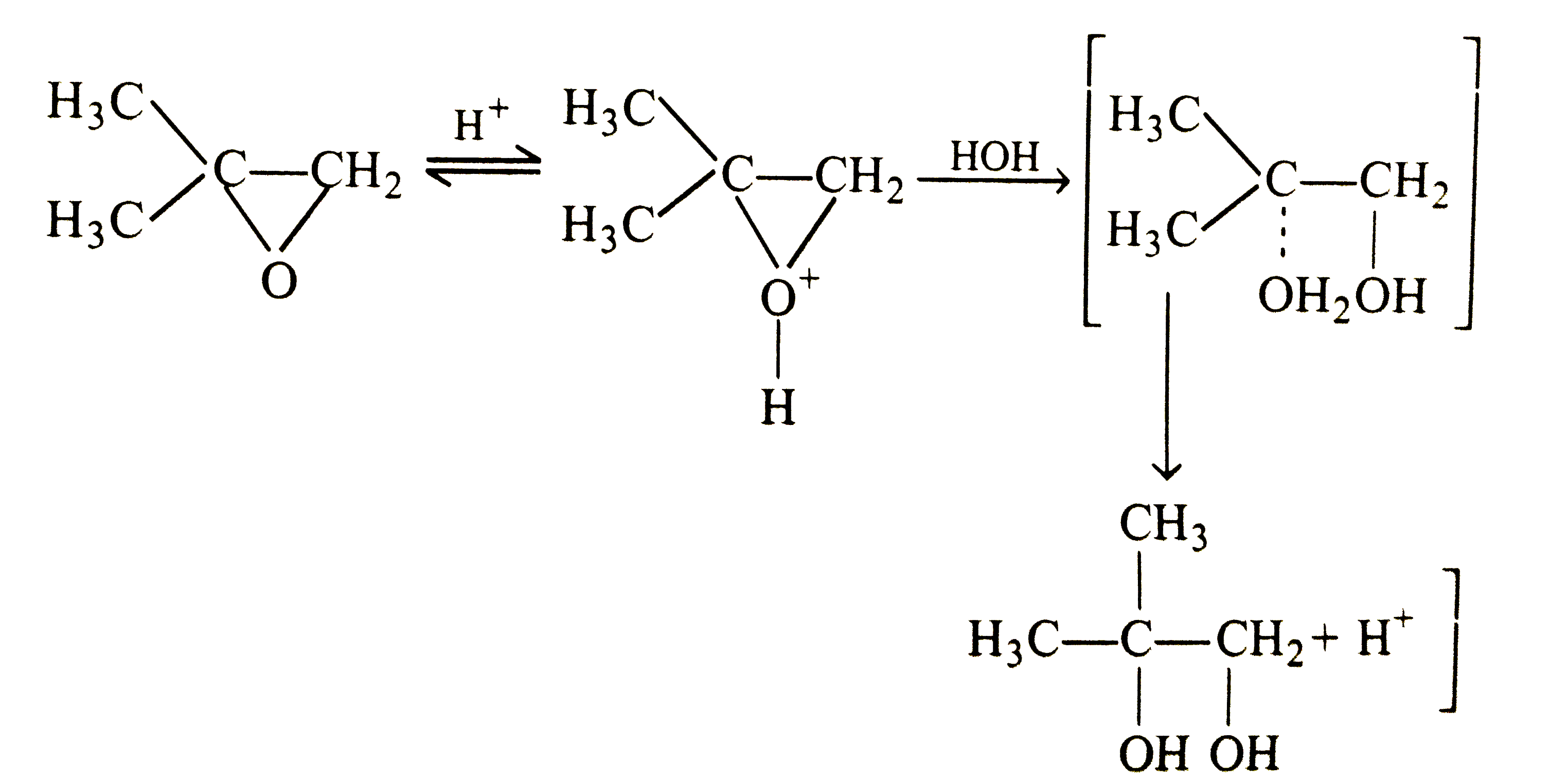Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise PROBLEMS BASED ON STRUCTURE AND PROPERTIES|7 VideosALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise BRAI STORMING PROBLEMS|32 VideosALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise PHENOLS|91 VideosALDEHYDES AND KETONES
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ALCOHOLS, PHENOLS AND ETHERS-ETHERS
- Give reason for the following : (i) (CH(3))(3)C-O-CH(3)underset("eth...
Text Solution
|
- (a) Discuss the structural relationship of water , alcohol and ethers....
Text Solution
|
- Account for the following : (i) Ethers have significant dipole momen...
Text Solution
|
- Answer the following : (i) What is the synthetic utility of the rea...
Text Solution
|
- How would you bring the following conversions ? (i) Ethyl alcohol to...
Text Solution
|
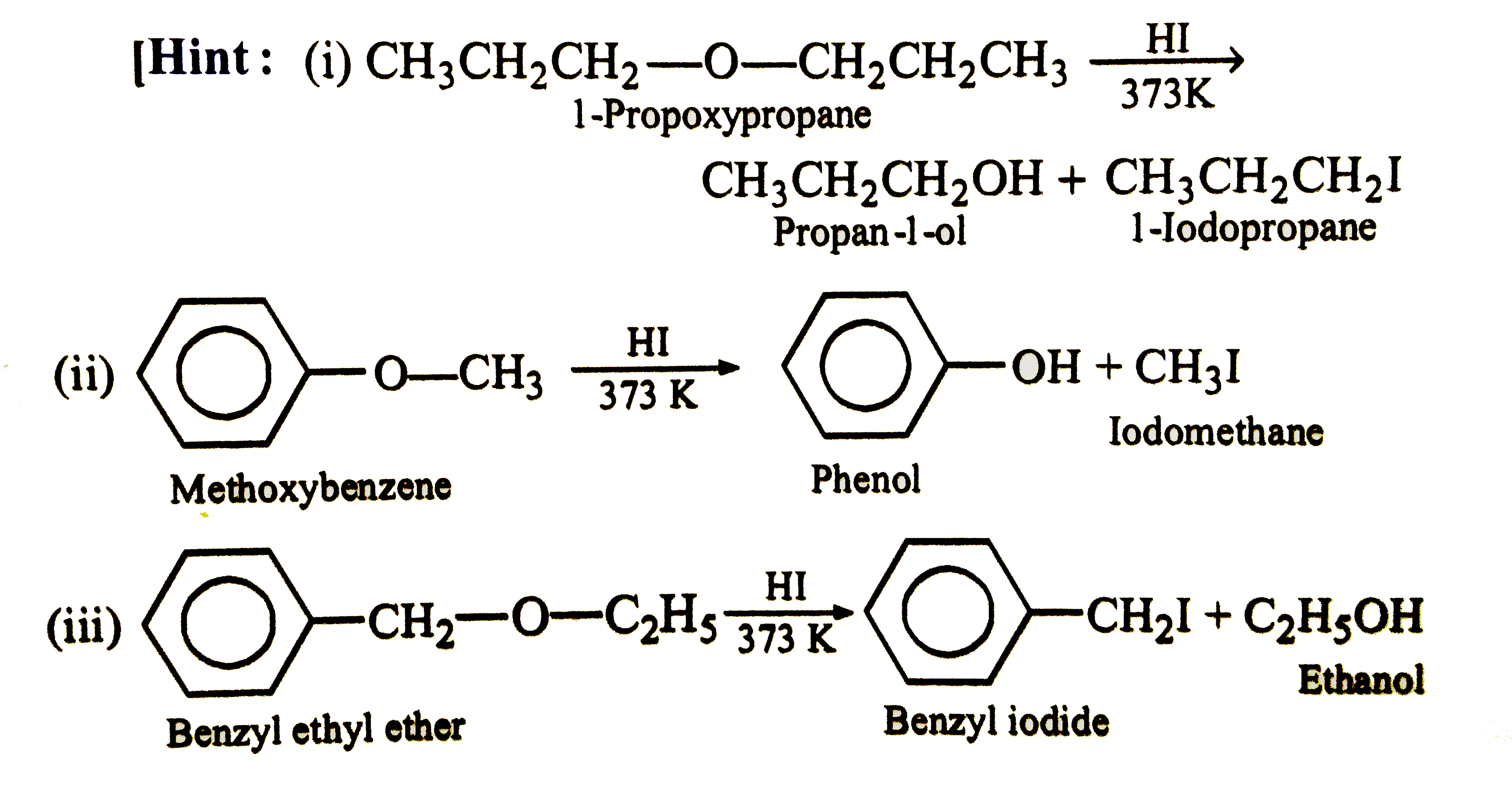
- Give the major product formed by heating the following ethers with con...
Text Solution
|
- Write the names of reagents and equations for the preparation of follo...
Text Solution
|
- Compound (A) C(4)H(10)O, is found to be soluble in sulphuric acid . (...
Text Solution
|
- A neutral compound (A) having C,H and O, on refluxing with HI yields m...
Text Solution
|
- (A) Ether behaves as bases in the presence of mineral acids. (R ) Du...
Text Solution
|
- (A ) The boiling point of ethanol is much higher than that of diethyl ...
Text Solution
|
- (A) Phenetole on clearvage with HI yields phenol and ethyl iodide (R...
Text Solution
|
- Assertion: Anisole undergoes eletctrophilic substitution at o-and p-po...
Text Solution
|
- (A) With HI, anisole forms iodobenzene and methyl alcohol. (R ) I^(-...
Text Solution
|
- (A) tert-Butyl methyl ether on cleavage with HI at 373K gives tert-but...
Text Solution
|
- (A) The C-O-C bond angle in ethers is higher than H-O-H bond angle in ...
Text Solution
|
- (A) tert-Butyl alcohol on heating with conc. H(2)SO(4) at 413K gives 2...
Text Solution
|
- (A) 1-Bromo-2,2-dimethyl propane on heating with ethanol gives 2-ethox...
Text Solution
|
- Assertion. t-Butyl Methyl ether is not prepared by the reaction of t-b...
Text Solution
|
- (A) Oxirane are cleaved under acidic and basic conditions. (R ) The ...
Text Solution
|