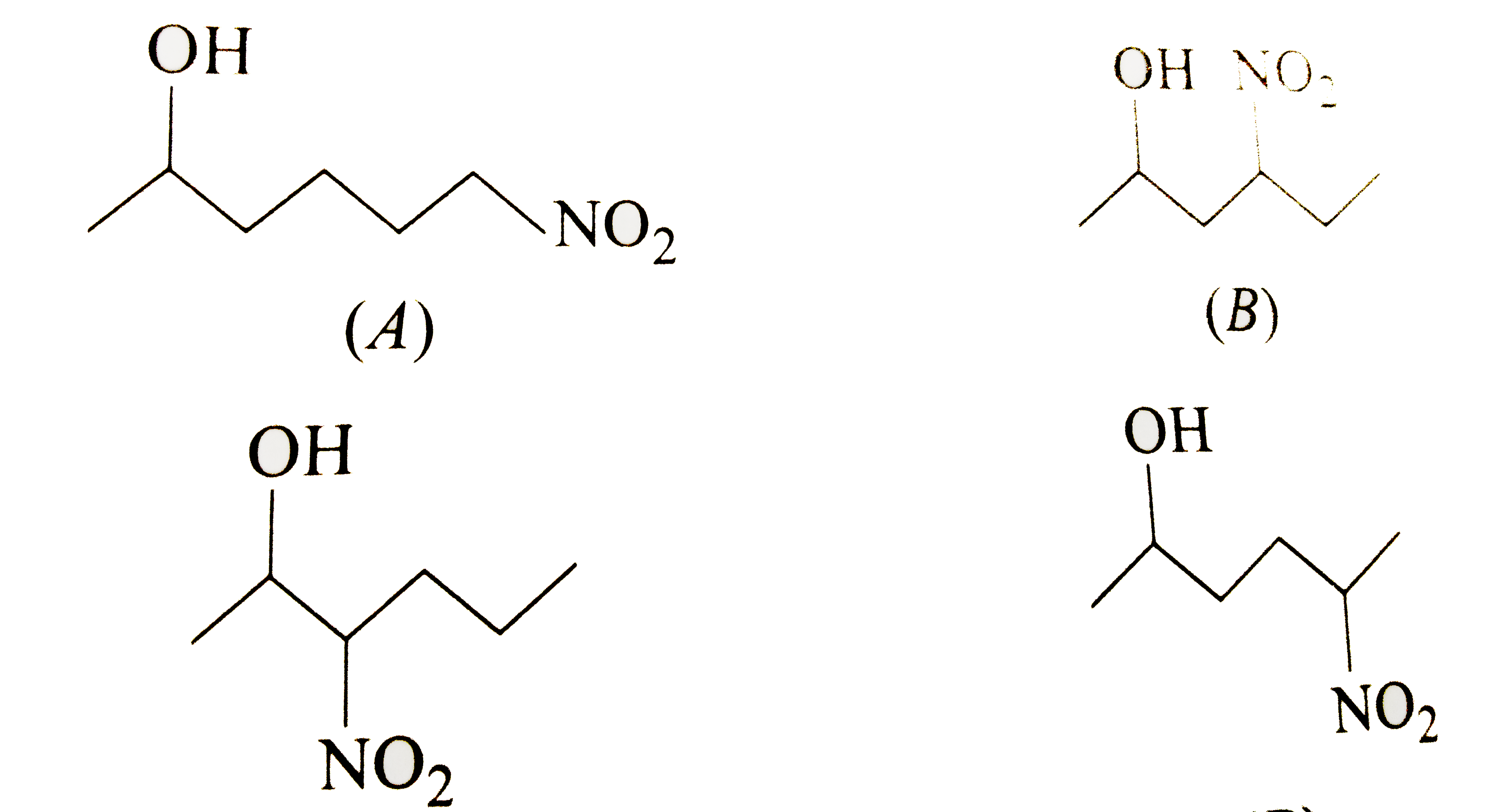
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise Passage 3|5 VideosALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise Passage 4|5 VideosALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise LINKED COMPREHENSION Passage 1|5 VideosALDEHYDES AND KETONES
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|10 Videos
OP TANDON-ALCOHOLS, PHENOLS AND ETHERS-passage 2
- Secondary and tertiary alcohols always give E(1) reaction in dehydrat...
Text Solution
|
- Secondary and tertiary alcohols always give E(1) reaction in dehydrat...
Text Solution
|
- Secondary and tertiary alcohols always give E(1) reaction in dehydrat...
Text Solution
|
- Secondary and tertiary alcohols always give E(1) reaction in dehydrat...
Text Solution
|
- Secondary and tertiary alcohols always give E(1) reaction in dehydrat...
Text Solution
|