A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
OP TANDON-ALCOHOLS, PHENOLS AND ETHERS-Passage 3
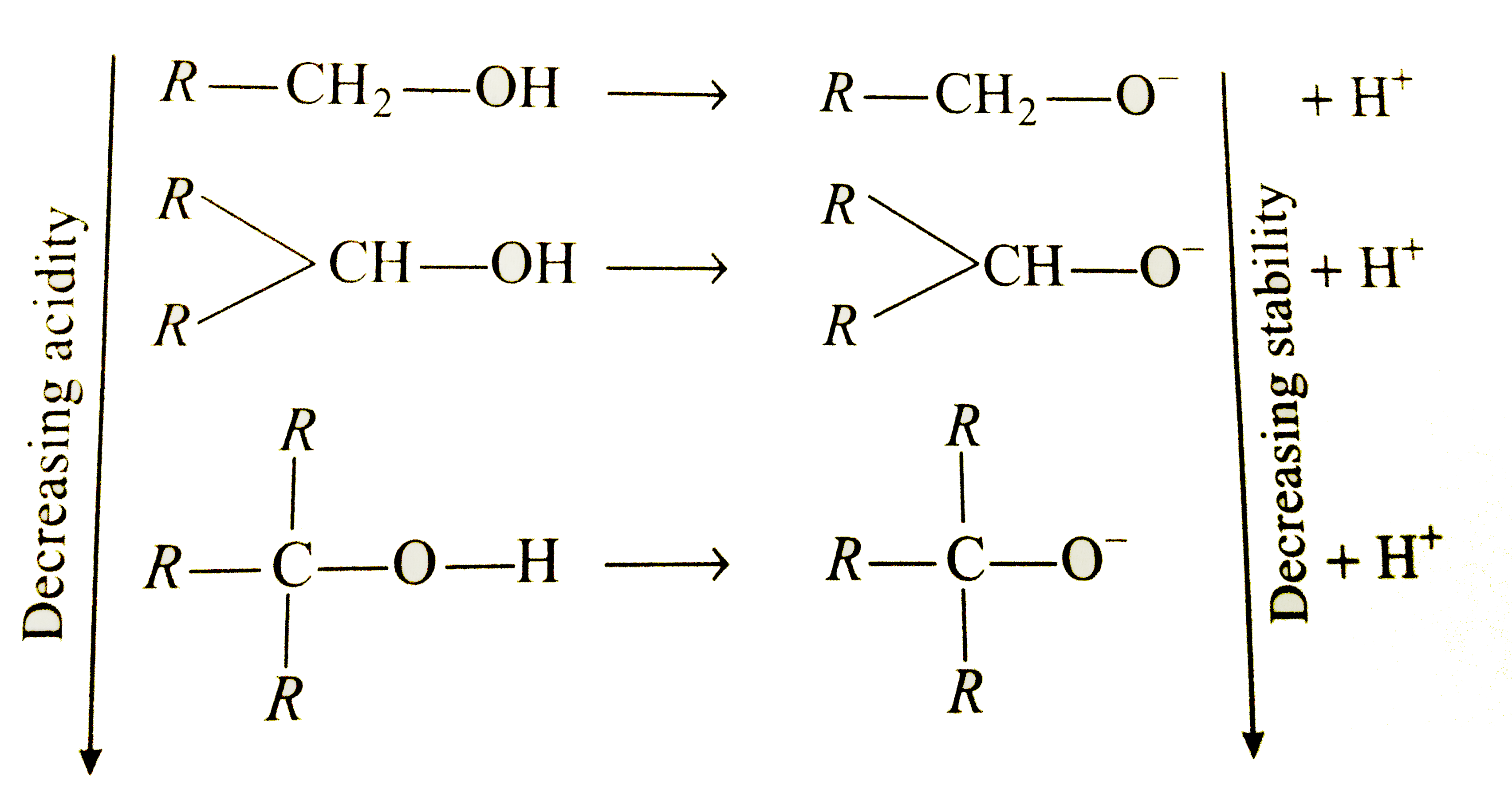
- Alcohols are acidic in nature because hydrogen is attached to oxygen w...
Text Solution
|
- Alcohols are acidic in nature because hydrogen is attached to oxygen w...
Text Solution
|
- Alcohols are acidic in nature because hydrogen is attached to oxygen w...
Text Solution
|
- Alcohols are acidic in nature because hydrogen is attached to oxygen w...
Text Solution
|
- Alcohols are acidic in nature because hydrogen is attached to oxygen w...
Text Solution
|