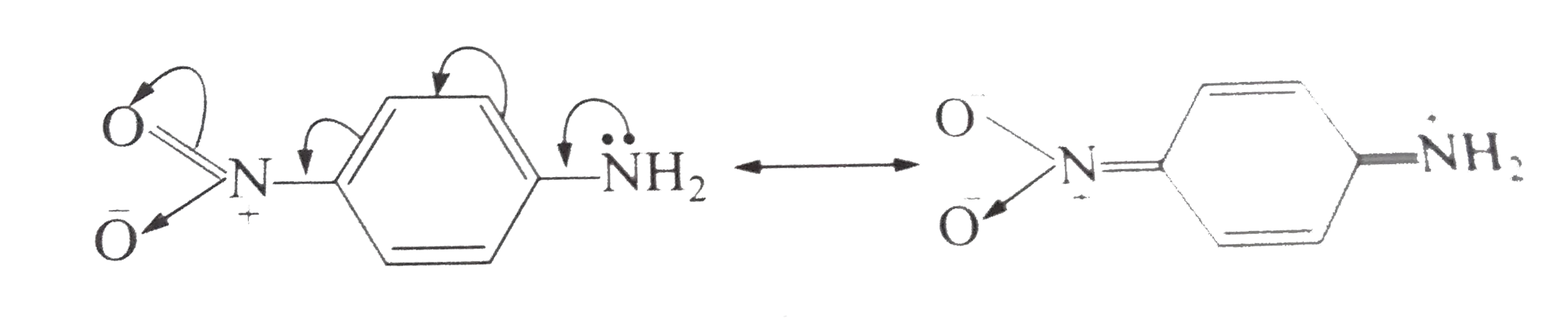Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise ILLUSTRATION|27 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise PROBLEMS FOR PRACTISE|17 VideosISOMERISM (STRUCTURAL AND STEROISOMERISM)
OP TANDON|Exercise INTEGER|7 VideosRADIOACTIVITY AND NUCLEAR TRANSFORMATION
OP TANDON|Exercise SECTION - VI|3 Videos
OP TANDON-ORGANIC COMPOUNDS CONTAINING NITROGEN-Single integer
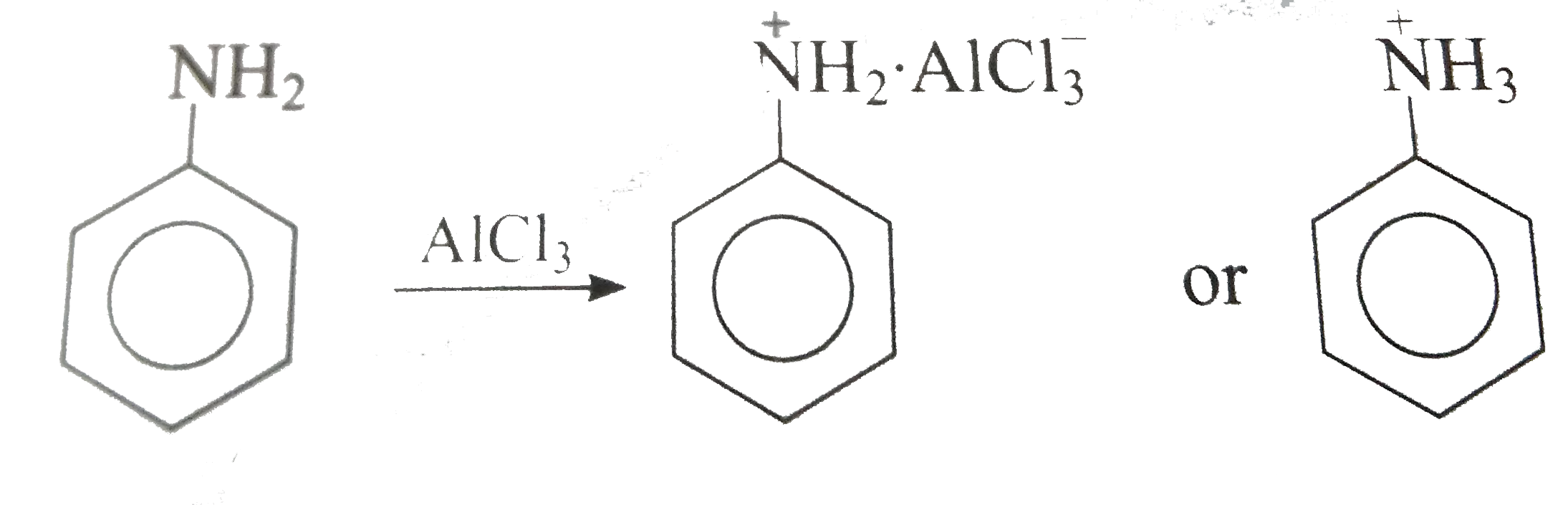
- Why p-nitroaniline is less basic then aniline (b) Aniline dissolves ...
Text Solution
|
- How many isomeric amines can have the formula C(4)H(11)N-
Text Solution
|
- How many of the following amines can give carbylamine reaction
Text Solution
|
- Of the following how many reactions are used for the preparation of am...
Text Solution
|
- Of the following how many can be separated by Hofmann's mustard oil re...
Text Solution
|
- Which of the following can be prepared by Gabriel method from their co...
Text Solution
|
- Examine the structural formula shown below and find out how many c...
Text Solution
|
- Find out number of reactions that are electrophilic aromatic subsituti...
Text Solution
|
- How many of the followin amines will unergo diazotisatio? Ethanamine...
Text Solution
|