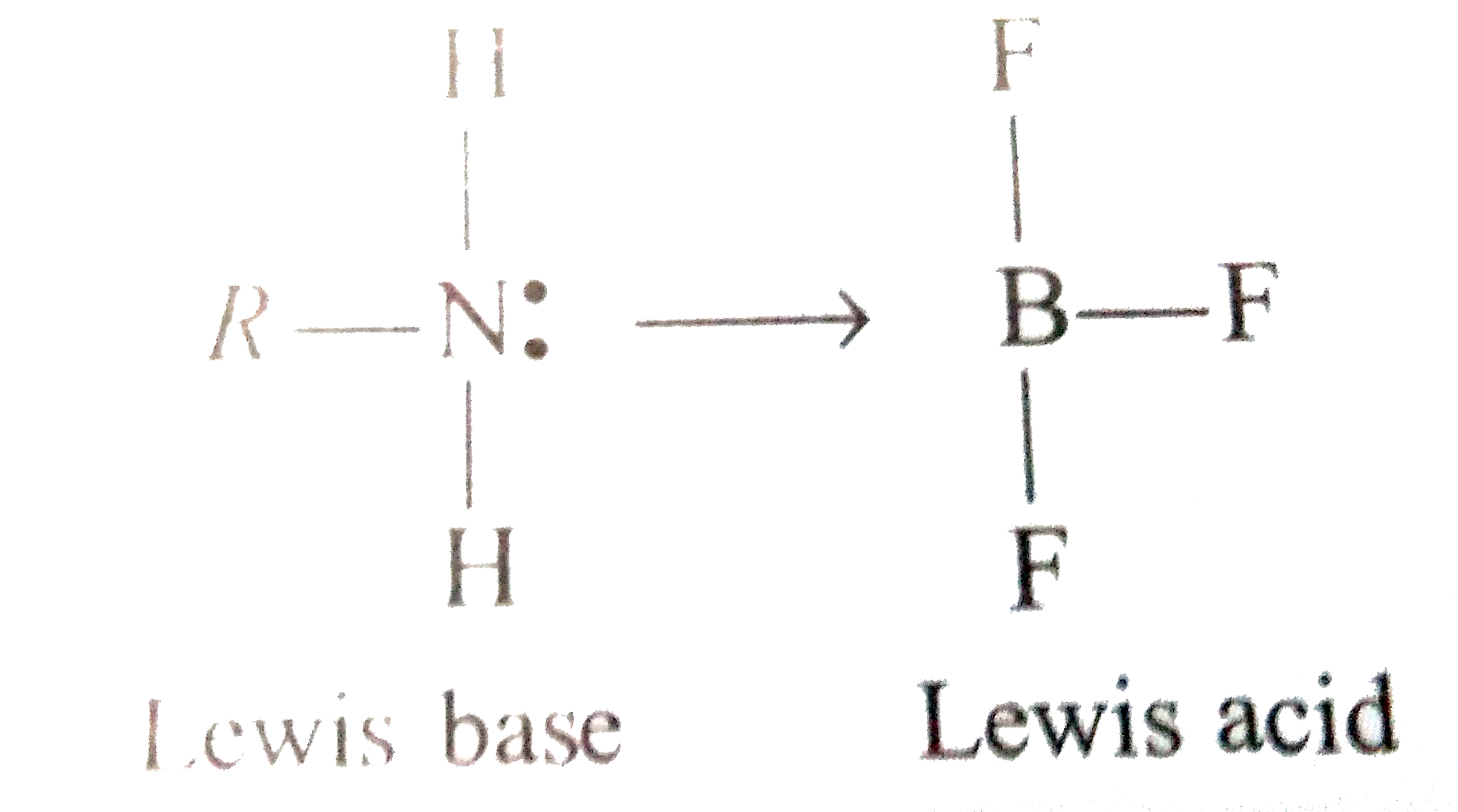A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise PASSAGE 4|3 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise PASSAGE 5|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise PASSAGE 2|5 VideosISOMERISM (STRUCTURAL AND STEROISOMERISM)
OP TANDON|Exercise INTEGER|7 VideosRADIOACTIVITY AND NUCLEAR TRANSFORMATION
OP TANDON|Exercise SECTION - VI|3 Videos
OP TANDON-ORGANIC COMPOUNDS CONTAINING NITROGEN-PASSAGE 3
- Amines are basic compounds They act as Lewis base due to the presence ...
Text Solution
|
- Amines are basic compounds They act as Lewis base due to the presence ...
Text Solution
|
- Amines are basic compounds They act as Lewis base due to the presence ...
Text Solution
|
- Amines are basic compounds They act as Lewis base due to the presence ...
Text Solution
|