A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 110-CHEMISTRY
- A solution contains 410.3 g H(2) SO(4) per litre of the solution at 20...
Text Solution
|
- Molten sodium chloride conducts electricity due to the presence of
Text Solution
|
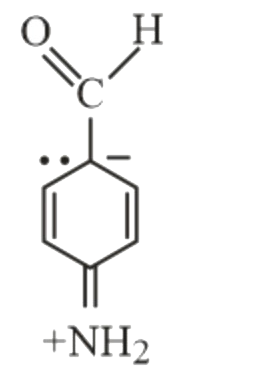
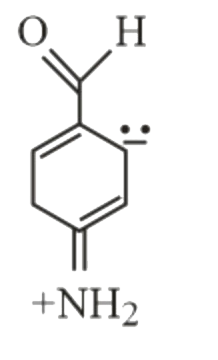
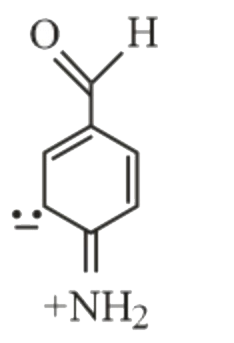
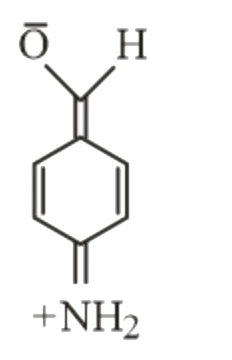
- Which of the following is not a resonance form of para-amino-benzaldeh...
Text Solution
|
- When the temperature is increased, surface tension of water:
Text Solution
|
- In the reaction sequence
Text Solution
|
- A + NaNO2 to N2O + NaCl + 2H2O in this reaction A can be
Text Solution
|
- 8:8 coordination of CsCl is found to change into 6:6 coordination :
Text Solution
|
- The compounds formed by the reaction of ammonia with chlorine and iodi...
Text Solution
|
- In the given reaction , overset(Br) overset(|)(CH2)-(CH2)(3)-CH2-OH ...
Text Solution
|
- Which of the following nitrate gives off different nitrogen oxide then...
Text Solution
|
- The pair in which phosphorus atoms have a formed oxidation state of +3...
Text Solution
|
- For the following cell, Zn(s)|ZnSO(4)(aq)||CuSO(4)(aq)||Cu(s) When...
Text Solution
|
- Which arrangement of the following esters correctly, indicates the dec...
Text Solution
|
- For an octahedral complex, which of the following d electron configura...
Text Solution
|
- In the reaction sequence CH -= CH + CH -=CH overset(Cu2Cl2)(rarr)(A)...
Text Solution
|
- Calcualate Delta(f)G^(@) "for" (NH(4)Cl,s) at 310 K. Given : Delta(...
Text Solution
|
- Among [Ni(CO)(4)], [NiBr4]^(2-), [Co(NH3)(4)Cl2]Cl, Na3[CoF6], BaO2 an...
Text Solution
|
- Which of the following can produce a colour change from yellowish brow...
Text Solution
|
- The number of waves made by a Bohr electron in an orbit of maximum mag...
Text Solution
|
- Which of the following combination of reagents does not undergo redox ...
Text Solution
|