A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ACIDS, BASES AND SALTS
DINESH PUBLICATION|Exercise SAQs SHORT ANSWER QUESTIONS|12 VideosACIDS, BASES AND SALTS
DINESH PUBLICATION|Exercise LAQs LONG ANSWER QUESTIONS|6 VideosACIDS, BASES AND SALTS
DINESH PUBLICATION|Exercise TEST YOUR KNOWLEDGE|19 VideosCARBON AND ITS COMPOUNDS
DINESH PUBLICATION|Exercise MCQs|29 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ACIDS, BASES AND SALTS -MULTIPLE CHOICE QUESTIONS
- Common salt besides being used in kitchen can also be used as the raw ...
Text Solution
|
- One of the constituents of baking powder is sodium hydrogen carbonate....
Text Solution
|
- To protect tooth decay, we are advised to brush out teeth regularly. T...
Text Solution
|
- Which of the following statements is correct about an aqueous solution...
Text Solution
|
- The pH of the gastric juices released during digestion is
Text Solution
|
- Which of the following phenomena occur when a small amount of acid is ...
Text Solution
|
- Which one of the following can be used as an acid base indicator by a ...
Text Solution
|
- Which of the following substances will not give carbon dioxide on trea...
Text Solution
|
- Which of the following is acidic in nature ?
Text Solution
|
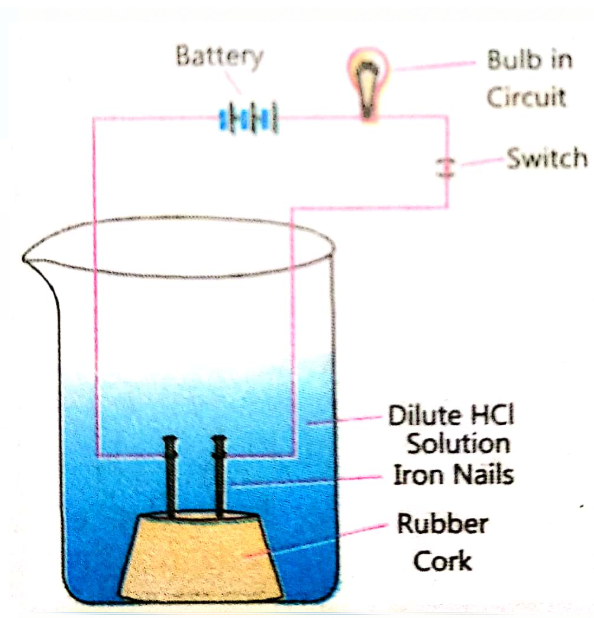
- In an attempt to demonstrate electrical conductivity through an electr...
Text Solution
|
- Which of the following is used for dissolution of gold ?
Text Solution
|
- Which of the following is not a mineral acid?
Text Solution
|
- Which of the following is not base ?
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
- Match the chemical substances given in column I with their appropriat...
Text Solution
|
- Equal volumes of hydrochloric acid and sodium hydroxide solutions of s...
Text Solution
|
- Which of the following is /are true when HCl (g) is passed through wat...
Text Solution
|
- Which of the following statement is true for acids?
Text Solution
|
- Which of the following are present in a dilute aqueous solution of hyd...
Text Solution
|
- Identify the correct representation of reaction occurring during chlor...
Text Solution
|