Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-THERMODYNAMICS-Exercise-1 Part-I Subjective question
- 2 mole of zinc is dissolved in HCl at 25^(@) C. The work done in open ...
Text Solution
|
- Classify the following processes as exothermic or endothermic: (A) B...
Text Solution
|
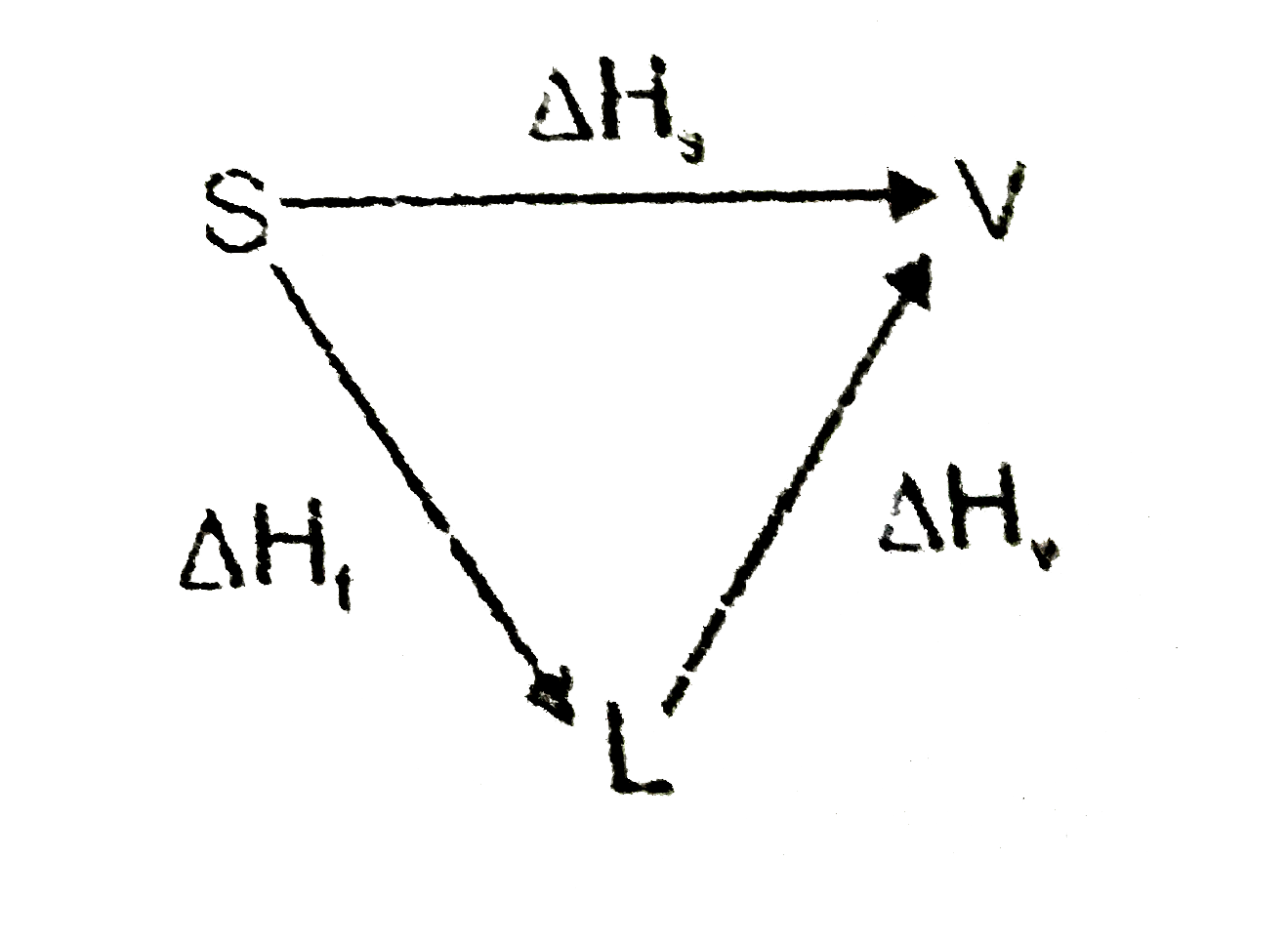
- Why is the enthalpy of sublimation equal to the sum of enthalpy of fus...
Text Solution
|
- For a chemical reaction, Delta C(p) is negative (Delta C(p) lt 0). T...
Text Solution
|
- Diborane is a potential rocket fuel which undergoes combustion accordi...
Text Solution
|
- The specific heats of iodine vapour and solid are 0.031 and 0.055 cal/...
Text Solution
|
- Predict the standard reaction enthalpy of 2NO(2)(g)rarrN(2)O(4)(g) at ...
Text Solution
|
- The heat of combustion of ethyl alcohol is -300 kcal. If the heats of ...
Text Solution
|
- Find out the heat evolved in combustion if 112 litres ( at STP) of wat...
Text Solution
|
- If {:(H(2)+1//2O(2)rarrH(2)O",",,,,DeltaH= -68 kcal),(K+H(2)OrarrKOH(a...
Text Solution
|
- Substance A(2)B(g) can undergoes decomposition to form two set of prod...
Text Solution
|
- One litre sample of a mixture of CH(4) and O(2) measured at 32^(@)C an...
Text Solution
|
- The standard enthalpy of decomposition of the yellow complex H(3)NSO(2...
Text Solution
|
- When 12.0g of carbon (graphite)reacted with oxygen to form CO and CO(2...
Text Solution
|
- Calculate the bond energy of Cl-Cl bond from the following data: CH(...
Text Solution
|
- Calculate DeltaH^(@) ("in" kJmol^(-1)) for the reaction CH(2)Cl(2)(...
Text Solution
|
- Calculate the enthalpy change (Delta H) of the following reaction 2C...
Text Solution
|
- Calculate change in enthalpy for the reaction at 27^(@)C H(2)(g)+Cl(...
Text Solution
|
- Estimate the average S-F bond enthalpy in SF(6). The values of standar...
Text Solution
|
- Calculate the standard enthalpy of solution of AgCl(s) in water DeltaH...
Text Solution
|