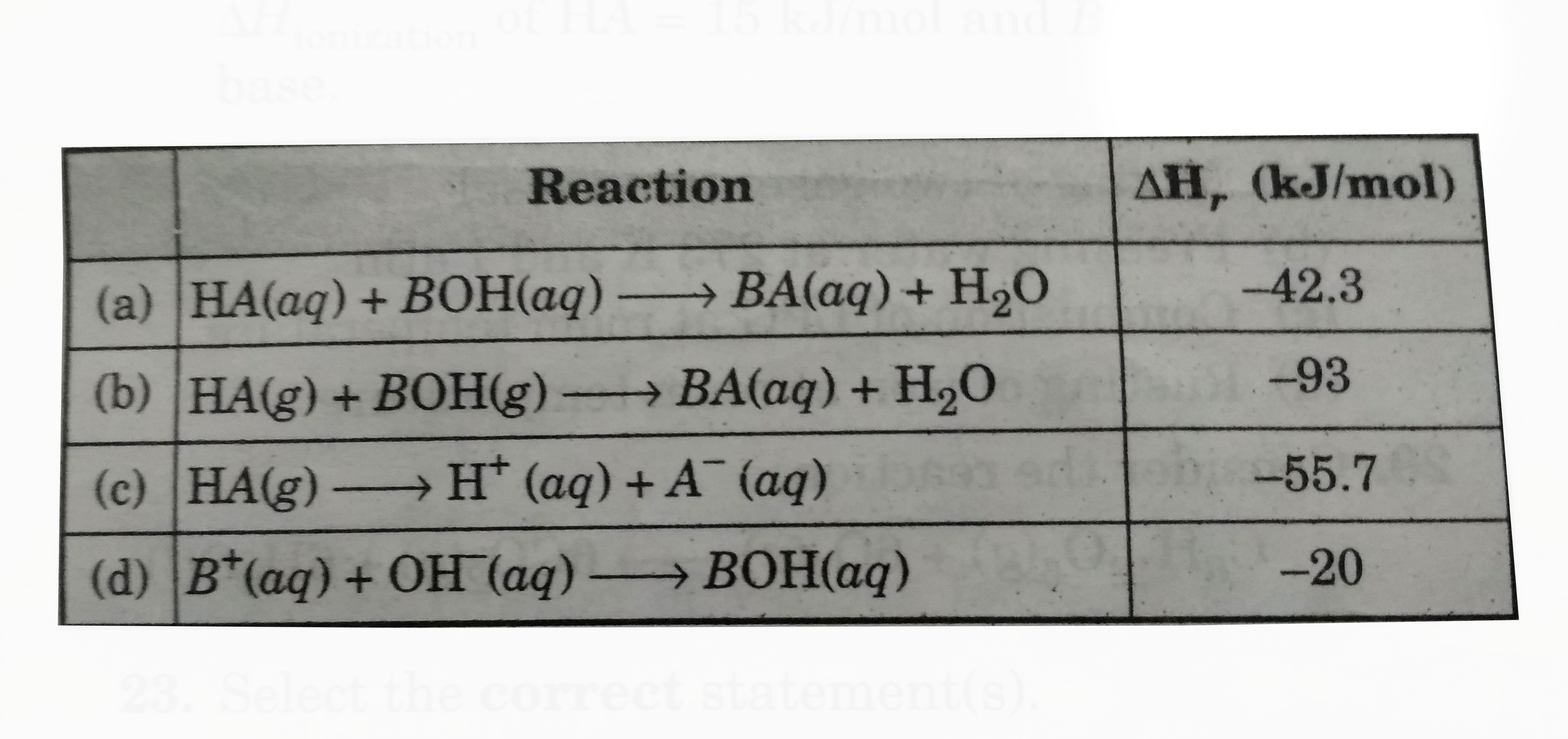
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE|Exercise exercise-3 part-III Advanced level Solutions (STAGE-I)|6 VideosTHERMODYNAMICS
RESONANCE|Exercise exercise-3 part-III Advanced level Solutions (STAGE-II)|45 VideosTHERMODYNAMICS
RESONANCE|Exercise exercise-3 part-1 Advanced level Solutions|30 VideosTEST SERIES
RESONANCE|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-THERMODYNAMICS-exercise-3 part-2 Advanced level Solutions
- Fixed amount of an ideal mono atomic gas contained in a sealed rigid v...
Text Solution
|
- Which of the following statement (s) is /are true?
Text Solution
|
- From the following date , mark the opation(s) where Delta H is correct...
Text Solution
|
- The value of DeltaH("transition") of C (graphite) rarr C (diamond) is ...
Text Solution
|
- Which of the following statement(s) is/are false?
Text Solution
|
- For the reaction 2Ag(2)O(s) rarr 4Ag(s) + O(2)(g), DeltaH is 61.17 KJ...
Text Solution
|
- Select the correct enthalpy at corresponding temperature using followi...
Text Solution
|
- Two moles of a perfect gas undergo the following processes: (a) a re...
Text Solution
|
- The enthalpy of combustion of mol. Wt. 180 glucose is -2808 KJ "mol"^(...
Text Solution
|
- A sample of certain mas s of an ideal polyatomic gas is expanded agai...
Text Solution
|
- The heat of combustion of acetylene is 312 Kcal . If heat of formatio...
Text Solution
|
- Boron exist in different allotropic forms. All allotropic form contain...
Text Solution
|
- 1 mole of an ideal gas is allowed to expand isothermally at 27^(@)C ti...
Text Solution
|
- Concerete is produced from a mixture of cement , water, sand and small...
Text Solution
|
- Concerete is produced from a mixture of cement , water, sand and small...
Text Solution
|
- Concerete is produced from a mixture of cement , water, sand and small...
Text Solution
|
- The accompanying diagram represents a reversible cannot cycle for an i...
Text Solution
|
- The accompanying diagram represents a reversible cannot cycle for an i...
Text Solution
|
- The accompanying diagram represents a reversible cannot cycle for an i...
Text Solution
|
- Match column-I to column-II standard entropy in KJ/k-molar at 25^(@)C ...
Text Solution
|