Text Solution
Verified by Experts
The correct Answer is:
RESONANCE-THERMODYNAMICS-exercise-3 part-III Advanced level Solutions (STAGE-II)
- A heat engine is a system that converts heat into mechanical work. A h...
Text Solution
|
- A heat engine is a system that converts heat into mechanical work. A h...
Text Solution
|
- One mole of Cl(2(g)) which may be assumed to obey the ideal gas law, i...
Text Solution
|
- For the following reaction occuring in dilute aqueous solution at 298K...
Text Solution
|
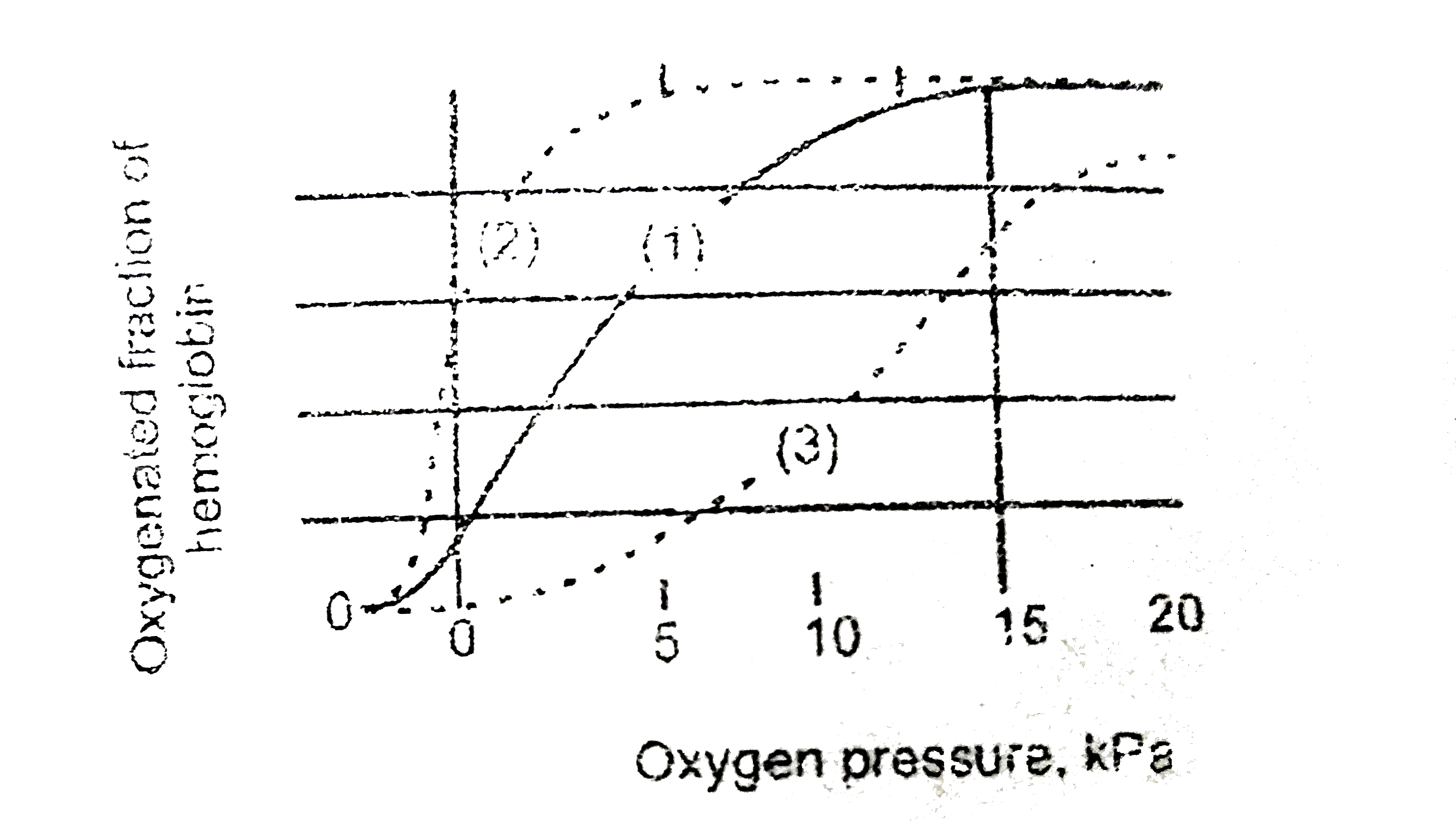
- Oxygen is of vital importance for all of us . Oxygen enters the body v...
Text Solution
|
- Since 1891 lighting lamps have been manufactured in the Netherlands. T...
Text Solution
|
- Since 1891 lighting lamps have been manufactured in the Netherlands. T...
Text Solution
|
- Since 1891 lighting lamps have been manufactured in the Netherlands. T...
Text Solution
|
- Since 1891 lighting lamps have been manufactured in the Netherlands. T...
Text Solution
|
- Since 1891 lighting lamps have been manufactured in the Netherlands. T...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|